Integrated safety and efficacy analysis of once-daily fluticasone furoate for the treatment of asthma
- PMID: 27881132
- PMCID: PMC5122018
- DOI: 10.1186/s12931-016-0473-x
Integrated safety and efficacy analysis of once-daily fluticasone furoate for the treatment of asthma
Abstract
Background: Fluticasone furoate is a once-daily inhaled corticosteroid. This report provides an overview of safety and efficacy data that support the use of once-daily fluticasone furoate 100 μg or 200 μg in adult and adolescent asthma patients.
Methods: Fourteen clinical studies (six Phase II and eight Phase III) were conducted as part of the fluticasone furoate global clinical development programme in asthma. Safety data from 10 parallel-group, randomised, double-blind Phase II and III studies (including 3345 patients who received at least one dose of fluticasone furoate) were integrated to provide information on adverse events, withdrawals, laboratory assessments, vital signs and hypothalamic-pituitary-adrenal axis function. The efficacy of once-daily fluticasone furoate was evaluated in all included studies.
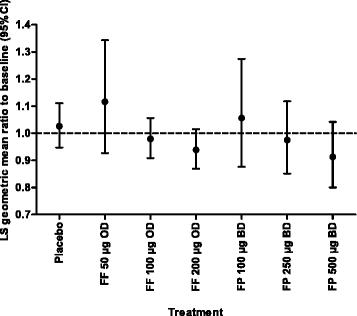
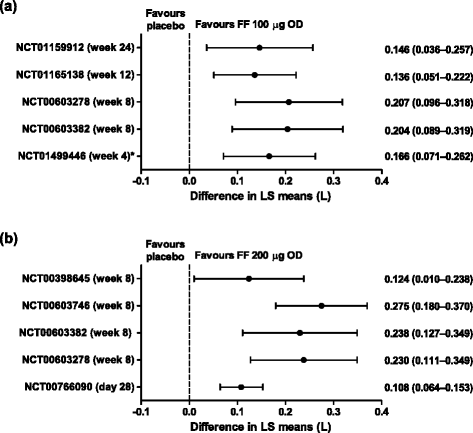
Results: Once-daily fluticasone furoate 100 μg and 200 μg safety profiles were consistent with those reported for other inhaled corticosteroids, and both doses consistently demonstrated efficacy versus placebo. In the integrated analysis, no dose-response relationship was observed for the overall incidence of adverse events and there were no significant effects of fluticasone furoate on hypothalamic-pituitary-adrenal axis function.
Conclusion: Once-daily fluticasone furoate 100 μg and 200 μg had acceptable safety profiles and was efficacious in adult and adolescent patients with asthma. There was no evidence of cortisol suppression at studied doses.
Trial registrations: GSK (NCT01499446/FFA20001, NCT00398645/FFA106783, NCT00766090/112202, NCT00603746/FFA109684, NCT00603278/FFA109685, NCT00603382/FFA109687, NCT01436071/115283, NCT01436110/115285, NCT01159912/112059, NCT01431950/114496, NCT01165138/HZA106827, NCT01086384/106837, NCT01134042/HZA106829 and NCT01244984/1139879).
Keywords: Adverse events; Cortisol suppression; Fluticasone furoate; Forced expiratory volume in one second; Inhaled corticosteroid; Integrated analysis; Safety.
Figures

References
-
- Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA). http://ginasthma.org/. Accessed 30 Sept 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

