Do emergency medicine journals promote trial registration and adherence to reporting guidelines? A survey of "Instructions for Authors"
- PMID: 27881175
- PMCID: PMC5121955
- DOI: 10.1186/s13049-016-0331-3
Do emergency medicine journals promote trial registration and adherence to reporting guidelines? A survey of "Instructions for Authors"
Abstract
Background: The aim of this study was to evaluate the current state of two publication practices, reporting guidelines requirements and clinical trial registration requirements, by analyzing the "Instructions for Authors" of emergency medicine journals.
Methods: We performed a web-based data abstraction from the "Instructions for Authors" of the 27 Emergency Medicine journals catalogued in the Expanded Science Citation Index of the 2014 Journal Citation Reports and Google Scholar Metrics h5-index to identify whether each journal required, recommended, or made no mention of the following reporting guidelines: EQUATOR Network, ICMJE, ARRIVE, CARE, CONSORT, STARD, TRIPOD, CHEERS, MOOSE, STROBE, COREQ, SRQR, SQUIRE, PRISMA-P, SPIRIT, PRISMA, and QUOROM. We also extracted whether journals required or recommended trial registration. Authors were blinded to one another's ratings until completion of the data validation. Cross-tabulations and descriptive statistics were calculated using IBM SPSS 22.
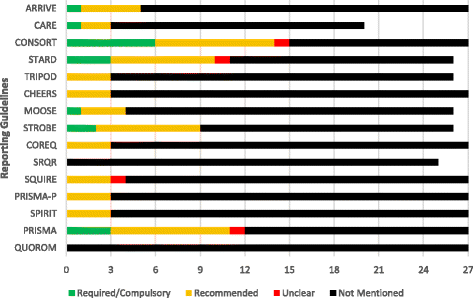
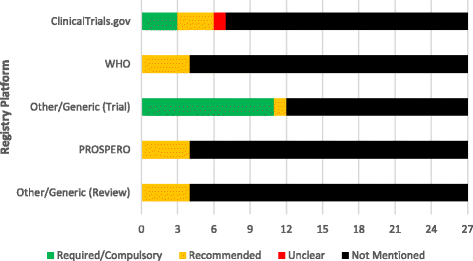
Results: Of the 27 emergency medicine journals, 11 (11/27, 40.7%) did not mention a single guideline within their "Instructions for Authors," while the remaining 16 (16/27, 59.3%) mentioned one or more guidelines. The QUOROM statement and SRQR were not mentioned by any journals whereas the ICMJE guidelines (18/27, 66.7%) and CONSORT statement (15/27, 55.6%) were mentioned most often. Of the 27 emergency medicine journals, 15 (15/27, 55.6%) did not mention trial or review registration, while the remaining 12 (12/27, 44.4%) at least mentioned one of the two. Trial registration through ClinicalTrials.gov was mentioned by seven (7/27, 25.9%) journals while the WHO registry was mentioned by four (4/27, 14.8%). Twelve (12/27, 44.4%) journals mentioned trial registration through any registry platform.
Discussion: The aim of this study was to evaluate the current state of two publication practices, reporting guidelines requirements and clinical trial registration requirements, by analyzing the "Instructions for Authors" of emergency medicine journals. In this study, there was not a single reporting guideline mentioned in more than half of the journals. This undermines efforts of other journals to improve the completeness and transparency of research reporting.
Conclusions: Reporting guidelines are infrequently required or recommended by emergency medicine journals. Furthermore, few require clinical trial registration. These two mechanisms may limit bias and should be considered for adoption by journal editors in emergency medicine.
Trial registration: UMIN000022486.
Keywords: CONSORT; Clinical trial registry; ClinicalTrials.gov; EQUATOR network; ICMJE; PRISMA; Reporting guidelines; STARD; STROBE; WHO.
Figures
Similar articles
-
Requirements for trial registration and adherence to reporting guidelines in critical care journals: a meta-epidemiological study of journals' instructions for authors.Int J Evid Based Healthc. 2018 Mar;16(1):55-65. doi: 10.1097/XEB.0000000000000120. Int J Evid Based Healthc. 2018. PMID: 28863029
-
An Evaluation of Reporting Guidelines and Clinical Trial Registry Requirements Among Addiction Medicine Journals.J Am Osteopath Assoc. 2020 Dec 1;120(12):823-830. doi: 10.7556/jaoa.2020.148. J Am Osteopath Assoc. 2020. PMID: 33075122
-
Hematology journals do not sufficiently adhere to reporting guidelines: a systematic review.J Thromb Haemost. 2017 Apr;15(4):608-617. doi: 10.1111/jth.13637. Epub 2017 Feb 27. J Thromb Haemost. 2017. PMID: 28122156
-
An Evaluation of Reporting Guidelines and Clinical Trial Registry Requirements Among Orthopaedic Surgery Journals.J Bone Joint Surg Am. 2018 Feb 7;100(3):e15. doi: 10.2106/JBJS.17.00529. J Bone Joint Surg Am. 2018. PMID: 29406351
-
Reporting Policies in Neurosurgical Journals: A Meta-Science Study of the Current State and Case for Standardization.World Neurosurg. 2022 Feb;158:11-23. doi: 10.1016/j.wneu.2021.10.143. Epub 2021 Oct 27. World Neurosurg. 2022. PMID: 34715370 Review.
Cited by
-
Has the reporting quality of published randomised controlled trial protocols improved since the SPIRIT statement? A methodological study.BMJ Open. 2020 Aug 26;10(8):e038283. doi: 10.1136/bmjopen-2020-038283. BMJ Open. 2020. PMID: 32847919 Free PMC article.
-
The relationship between endorsing reporting guidelines or trial registration and the impact factor or total citations in surgical journals.PeerJ. 2022 Jan 25;10:e12837. doi: 10.7717/peerj.12837. eCollection 2022. PeerJ. 2022. PMID: 35127293 Free PMC article. Review.
-
Methodological Rigor and Temporal Trends of Cardiovascular Medicine Meta-Analyses in Highest-Impact Journals.J Am Heart Assoc. 2021 Sep 21;10(18):e021367. doi: 10.1161/JAHA.121.021367. Epub 2021 Sep 17. J Am Heart Assoc. 2021. PMID: 34533035 Free PMC article.
-
A cross-sectional study on the endorsement of reporting guidelines and clinical trial registration among immunology and allergy journals.PLoS One. 2025 May 7;20(5):e0322003. doi: 10.1371/journal.pone.0322003. eCollection 2025. PLoS One. 2025. PMID: 40333833 Free PMC article.
-
Endorsement of reporting guidelines and clinical trial registration across Scopus-indexed rheumatology journals: a cross-sectional analysis.Rheumatol Int. 2024 May;44(5):909-917. doi: 10.1007/s00296-023-05474-4. Epub 2023 Oct 20. Rheumatol Int. 2024. PMID: 37861727
References
-
- Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006;185:263–267. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

