Kinetics of Meningococcal Serogroup C-Specific Functional Antibody Levels Up to 15 Years after a Single Immunization with a Meningococcal Serogroup C Conjugate Vaccine during Adolescence
- PMID: 27881489
- PMCID: PMC5299121
- DOI: 10.1128/CVI.00429-16
Kinetics of Meningococcal Serogroup C-Specific Functional Antibody Levels Up to 15 Years after a Single Immunization with a Meningococcal Serogroup C Conjugate Vaccine during Adolescence
Abstract
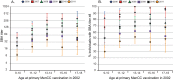
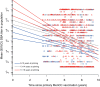
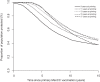
Adolescent vaccination is now considered the key factor for offering direct protection against meningococcal disease but also for reducing carriage and transmission and, in this way, establishing herd protection. This study estimated age-dependent patterns in functional meningococcal serogroup C (MenC) antibody kinetics after primary MenC conjugate (MenCC) vaccination in adolescents. Serum samples (n = 1,676) were drawn from 2006 to 2011 from individuals aged 9 to 18 years at the time of primary MenCC vaccination in 2002. Functional antibody levels were measured with a serum bactericidal antibody assay (SBA) using rabbit complement. SBA titers gradually declined with time. Up to 9 years after primary vaccination, SBA titers were estimated to be higher in individuals who were aged 13 to 18 years at priming than in those who were aged 9 to 10 years at priming. Based on a linear mixed model, the higher functional antibody levels with age seem to be due to the achievement of higher peak levels upon vaccination rather than to lower rates of decline. It is estimated that 35 to 50% of individuals who received a single primary MenCC vaccination at an age of 9 to 18 years in 2002 will still have sufficient protective antibody levels 15 years later. Using a linear mixed model based on cohort data for a single dated serum sample per person, we were able to estimate the level of protection against MenC up to 15 years after a single vaccination. The current study shows that analysis of antibody kinetics can be done using cross-sectional serology data and is therefore relevant for future serosurveillance studies.
Keywords: Neisseria meningitidis; adolescent; antibodies; long-term; meningococcal serogroup C conjugate vaccine.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Long-term persistence of protective antibodies in Dutch adolescents following a meningococcal serogroup C tetanus booster vaccination.Vaccine. 2016 Dec 7;34(50):6309-6315. doi: 10.1016/j.vaccine.2016.10.049. Epub 2016 Nov 4. Vaccine. 2016. PMID: 27817957
-
Persistence of serum bactericidal antibody one year after a booster dose of either a glycoconjugate or a plain polysaccharide vaccine against serogroup C Neisseria meningitidis given to adolescents previously immunized with a glycoconjugate vaccine.Pediatr Infect Dis J. 2011 Nov;30(11):e203-8. doi: 10.1097/INF.0b013e318224fb14. Pediatr Infect Dis J. 2011. PMID: 21673612 Clinical Trial.
-
Antibody persistence after serogroup C meningococcal conjugate vaccine in children with sickle cell disease.Vaccine. 2016 Aug 5;34(36):4327-34. doi: 10.1016/j.vaccine.2016.06.072. Epub 2016 Jul 6. Vaccine. 2016. PMID: 27395566
-
Effectiveness and duration of protection of primary and booster immunisation against meningococcal serogroup C disease with meningococcal conjugate C and ACWY vaccines: Systematic review.J Infect. 2024 Sep;89(3):106228. doi: 10.1016/j.jinf.2024.106228. Epub 2024 Jul 10. J Infect. 2024. PMID: 38996818
-
Is a single infant priming dose of meningococcal serogroup C conjugate vaccine in the United Kingdom sufficient?Hum Vaccin Immunother. 2015;11(6):1501-6. doi: 10.1080/21645515.2015.1019189. Hum Vaccin Immunother. 2015. PMID: 25912095 Free PMC article. Review.
References
-
- Neppelenbroek SE, de Vries M, de Greeff SC, Timen A. 2003. “Da's goed gedaan?” Report of the Dutch national vaccination campaign against meningococcal serogroup C disease in 2002. Municipal and Regional Health Service (GGD), Utrecht, The Netherlands.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

