Cardiac T-Tubule Microanatomy and Function
- PMID: 27881552
- PMCID: PMC6151489
- DOI: 10.1152/physrev.00037.2015
Cardiac T-Tubule Microanatomy and Function
Abstract
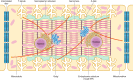
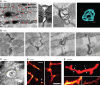
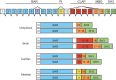
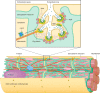
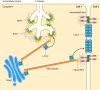
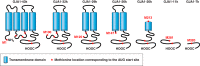
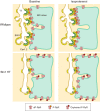
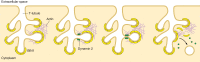
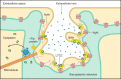
Unique to striated muscle cells, transverse tubules (t-tubules) are membrane organelles that consist of sarcolemma penetrating into the myocyte interior, forming a highly branched and interconnected network. Mature t-tubule networks are found in mammalian ventricular cardiomyocytes, with the transverse components of t-tubules occurring near sarcomeric z-discs. Cardiac t-tubules contain membrane microdomains enriched with ion channels and signaling molecules. The microdomains serve as key signaling hubs in regulation of cardiomyocyte function. Dyad microdomains formed at the junctional contact between t-tubule membrane and neighboring sarcoplasmic reticulum are critical in calcium signaling and excitation-contraction coupling necessary for beat-to-beat heart contraction. In this review, we provide an overview of the current knowledge in gross morphology and structure, membrane and protein composition, and function of the cardiac t-tubule network. We also review in detail current knowledge on the formation of functional membrane subdomains within t-tubules, with a particular focus on the cardiac dyad microdomain. Lastly, we discuss the dynamic nature of t-tubules including membrane turnover, trafficking of transmembrane proteins, and the life cycles of membrane subdomains such as the cardiac BIN1-microdomain, as well as t-tubule remodeling and alteration in diseased hearts. Understanding cardiac t-tubule biology in normal and failing hearts is providing novel diagnostic and therapeutic opportunities to better treat patients with failing hearts.
Copyright © 2017 the American Physiological Society.
Figures
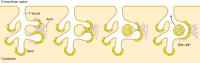
References
-
- Asghari P, Scriven DR, Sanatani S, Gandhi SK, Campbell AI, Moore ED. Nonuniform and variable arrangements of ryanodine receptors within mammalian ventricular couplons. Circ Res : 252–262, 2014. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

