Plasma Metabolomics Implicates Modified Transfer RNAs and Altered Bioenergetics in the Outcomes of Pulmonary Arterial Hypertension
- PMID: 27881557
- PMCID: PMC5287439
- DOI: 10.1161/CIRCULATIONAHA.116.024602
Plasma Metabolomics Implicates Modified Transfer RNAs and Altered Bioenergetics in the Outcomes of Pulmonary Arterial Hypertension
Abstract
Background: Pulmonary arterial hypertension (PAH) is a heterogeneous disorder with high mortality.
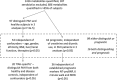
Methods: We conducted a comprehensive study of plasma metabolites using ultraperformance liquid chromatography mass spectrometry to identify patients at high risk of early death, to identify patients who respond well to treatment, and to provide novel molecular insights into disease pathogenesis.
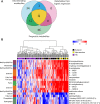
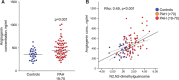
Results: Fifty-three circulating metabolites distinguished well-phenotyped patients with idiopathic or heritable PAH (n=365) from healthy control subjects (n=121) after correction for multiple testing (P<7.3e-5) and confounding factors, including drug therapy, and renal and hepatic impairment. A subset of 20 of 53 metabolites also discriminated patients with PAH from disease control subjects (symptomatic patients without pulmonary hypertension, n=139). Sixty-two metabolites were prognostic in PAH, with 36 of 62 independent of established prognostic markers. Increased levels of tRNA-specific modified nucleosides (N2,N2-dimethylguanosine, N1-methylinosine), tricarboxylic acid cycle intermediates (malate, fumarate), glutamate, fatty acid acylcarnitines, tryptophan, and polyamine metabolites and decreased levels of steroids, sphingomyelins, and phosphatidylcholines distinguished patients from control subjects. The largest differences correlated with increased risk of death, and correction of several metabolites over time was associated with a better outcome. Patients who responded to calcium channel blocker therapy had metabolic profiles similar to those of healthy control subjects.
Conclusions: Metabolic profiles in PAH are strongly related to survival and should be considered part of the deep phenotypic characterization of this disease. Our results support the investigation of targeted therapeutic strategies that seek to address the alterations in translational regulation and energy metabolism that characterize these patients.
Keywords: hypertension, pulmonary; metabolism; metabolome; metabolomics; pulmonary circulation.
© 2016 The Authors.
Figures




References
-
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. - PubMed
-
- Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. - PubMed
-
- Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension, part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation. 2015;131:1691–1702. doi: 10.1161/CIRCULATIONAHA.114.006979. - PMC - PubMed
-
- Maron BA, Leopold JA. Emerging concepts in the molecular basis of pulmonary arterial hypertension, part II: neurohormonal signaling contributes to the pulmonary vascular and right ventricular pathophenotype of pulmonary arterial hypertension. Circulation. 2015;131:2079–2091. doi: 10.1161/CIRCULATIONAHA.114.006980. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

