GTSE1 tunes microtubule stability for chromosome alignment and segregation by inhibiting the microtubule depolymerase MCAK
- PMID: 27881713
- PMCID: PMC5147003
- DOI: 10.1083/jcb.201606081
GTSE1 tunes microtubule stability for chromosome alignment and segregation by inhibiting the microtubule depolymerase MCAK
Abstract
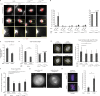
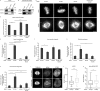
The dynamic regulation of microtubules (MTs) during mitosis is critical for accurate chromosome segregation and genome stability. Cancer cell lines with hyperstabilized kinetochore MTs have increased segregation errors and elevated chromosomal instability (CIN), but the genetic defects responsible remain largely unknown. The MT depolymerase MCAK (mitotic centromere-associated kinesin) can influence CIN through its impact on MT stability, but how its potent activity is controlled in cells remains unclear. In this study, we show that GTSE1, a protein found overexpressed in aneuploid cancer cell lines and tumors, regulates MT stability during mitosis by inhibiting MCAK MT depolymerase activity. Cells lacking GTSE1 have defects in chromosome alignment and spindle positioning as a result of MT instability caused by excess MCAK activity. Reducing GTSE1 levels in CIN cancer cell lines reduces chromosome missegregation defects, whereas artificially inducing GTSE1 levels in chromosomally stable cells elevates chromosome missegregation and CIN. Thus, GTSE1 inhibition of MCAK activity regulates the balance of MT stability that determines the fidelity of chromosome alignment, segregation, and chromosomal stability.
© 2016 Bendre et al.
Figures






References
-
- Asteriti I.A., Rensen W.M., Lindon C., Lavia P., and Guarguaglini G.. 2010. The Aurora-A/TPX2 complex: a novel oncogenic holoenzyme? Biochim. Biophys. Acta. 1806:230–239. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

