A Biophysical Model for Cytotoxic Cell Swelling
- PMID: 27881775
- PMCID: PMC6604918
- DOI: 10.1523/JNEUROSCI.1934-16.2016
A Biophysical Model for Cytotoxic Cell Swelling
Abstract
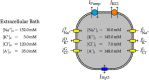
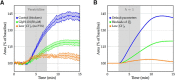
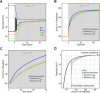
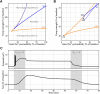
We present a dynamic biophysical model to explain neuronal swelling underlying cytotoxic edema in conditions of low energy supply, as observed in cerebral ischemia. Our model contains Hodgkin-Huxley-type ion currents, a recently discovered voltage-gated chloride flux through the ion exchanger SLC26A11, active KCC2-mediated chloride extrusion, and ATP-dependent pumps. The model predicts changes in ion gradients and cell swelling during ischemia of various severity or channel blockage with realistic timescales. We theoretically substantiate experimental observations of chloride influx generating cytotoxic edema, while sodium entry alone does not. We show a tipping point of Na+/K+-ATPase functioning, where below cell volume rapidly increases as a function of the remaining pump activity, and a Gibbs-Donnan-like equilibrium state is reached. This precludes a return to physiological conditions even when pump strength returns to baseline. However, when voltage-gated sodium channels are temporarily blocked, cell volume and membrane potential normalize, yielding a potential therapeutic strategy.
Significance statement: Cytotoxic edema most commonly results from energy shortage, such as in cerebral ischemia, and refers to the swelling of brain cells due to the entry of water from the extracellular space. We show that the principle of electroneutrality explains why chloride influx is essential for the development of cytotoxic edema. With the help of a biophysical model of a single neuron, we show that a tipping point of the energy supply exists, below which the cell volume rapidly increases. We simulate realistic time courses to and reveal critical components of neuronal swelling in conditions of low energy supply. Furthermore, we show that, after transient blockade of the energy supply, cytotoxic edema may be reversed by temporary blockade of Na+ channels.
Keywords: ATP; Gibbs–Donnan equilibrium; cytotoxic edema; electrodiffusion; osmosis.
Copyright © 2016 the authors 0270-6474/16/3611881-10$15.00/0.
Figures



Similar articles
-
The cellular mechanisms of neuronal swelling underlying cytotoxic edema.Cell. 2015 Apr 23;161(3):610-621. doi: 10.1016/j.cell.2015.03.029. Cell. 2015. PMID: 25910210
-
Volume changes in neurons: hyperexcitability and neuronal death.Contrib Nephrol. 2006;152:221-240. doi: 10.1159/000096326. Contrib Nephrol. 2006. PMID: 17065815 Review.
-
Palytoxin-induced channel formation within the Na+/K+-ATPase does not require a catalytically active enzyme.Eur J Biochem. 1997 Sep 15;248(3):717-23. doi: 10.1111/j.1432-1033.1997.00717.x. Eur J Biochem. 1997. PMID: 9342222
-
Octanoic acid inhibits astrocyte volume control: implications for cerebral edema in Reye's syndrome.J Neurochem. 1989 Apr;52(4):1197-202. doi: 10.1111/j.1471-4159.1989.tb01866.x. J Neurochem. 1989. PMID: 2538563
-
Molecular control of cardiac sodium homeostasis in health and disease.J Cardiovasc Electrophysiol. 2006 May;17 Suppl 1:S47-S56. doi: 10.1111/j.1540-8167.2006.00383.x. J Cardiovasc Electrophysiol. 2006. PMID: 16686682 Review.
Cited by
-
Posterior Reversible Encephalopathy Syndrome (PRES): Pathophysiology and Neuro-Imaging.Front Neurol. 2020 Jun 16;11:463. doi: 10.3389/fneur.2020.00463. eCollection 2020. Front Neurol. 2020. PMID: 32612567 Free PMC article. Review.
-
INF2-mediated actin filament reorganization confers intrinsic resilience to neuronal ischemic injury.Nat Commun. 2022 Oct 13;13(1):6037. doi: 10.1038/s41467-022-33268-y. Nat Commun. 2022. PMID: 36229429 Free PMC article.
-
Rapid Fluorescence Lifetime Imaging Reveals That TRPV4 Channels Promote Dysregulation of Neuronal Na+ in Ischemia.J Neurosci. 2022 Jan 26;42(4):552-566. doi: 10.1523/JNEUROSCI.0819-21.2021. Epub 2021 Dec 6. J Neurosci. 2022. PMID: 34872928 Free PMC article.
-
Neuroprotective Treatment of Postanoxic Encephalopathy: A Review of Clinical Evidence.Front Neurol. 2021 Feb 18;12:614698. doi: 10.3389/fneur.2021.614698. eCollection 2021. Front Neurol. 2021. PMID: 33679581 Free PMC article. Review.
-
An electrodiffusive neuron-extracellular-glia model for exploring the genesis of slow potentials in the brain.PLoS Comput Biol. 2021 Jul 16;17(7):e1008143. doi: 10.1371/journal.pcbi.1008143. eCollection 2021 Jul. PLoS Comput Biol. 2021. PMID: 34270543 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
