Systemic and mucosal immune responses following oral adenoviral delivery of influenza vaccine to the human intestine by radio controlled capsule
- PMID: 27881837
- PMCID: PMC5121599
- DOI: 10.1038/srep37295
Systemic and mucosal immune responses following oral adenoviral delivery of influenza vaccine to the human intestine by radio controlled capsule
Abstract
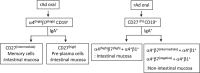
There are several benefits of oral immunization including the ability to elicit mucosal immune responses that may protect against pathogens that invade through a mucosal surface. Our understanding of human immune biology is hampered by the difficulty in isolating mucosal cells from humans, and the fact that animal models may or may not completely mirror human intestinal immunobiology. In this human pharmacodynamic study, a novel adenovirus vector-based platform expressing influenza hemagglutinin was explored. We used radio-controlled capsules to deliver the vaccine to either the jejunum or the ileum. The resulting immune responses induced by immunization at each of the intestinal sites were investigated. Both intestinal sites were capable of inducing mucosal and systemic immune responses to influenza hemagglutinin, but ileum delivery induced higher numbers of antibody secreting cells of IgG and IgA isotypes, increased mucosal homing B cells, and higher number of vaccine responders. Overall, these data provided substantial insights into human mucosal inductive sites, and aided in the design and selection of indications that could be used with this oral vaccine platform.
Conflict of interest statement
The authors LK, CJM, KAH, JRB, GRT, DL, and SNT are employees and/or own stock in Vaxart, the sponsor of the study. The authors EPS and WJD work for a contract research organization that ran the study for the sponsor.
Figures






References
-
- Ogra P. L., Fishaut M. & Gallagher M. R. Viral vaccination via the mucosal routes. Rev Infect Dis 2, 352–369 (1980). - PubMed
-
- Ermund A., Schutte A., Johansson M. E., Gustafsson J. K. & Hansson G. C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol 305, G341–G347, doi: 10.1152/ajpgi.00046.2013 (2013). - DOI - PMC - PubMed
-
- Van Kruiningen H. J., West A. B., Freda B. J. & Holmes K. A. Distribution of Peyer’s patches in the distal ileum. Inflamm Bowel Dis 8, 180–185 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

