Netrin-1-Induced Stem Cell Bioactivity Contributes to the Regeneration of Injured Tissues via the Lipid Raft-Dependent Integrin α6β4 Signaling Pathway
- PMID: 27881869
- PMCID: PMC5121594
- DOI: 10.1038/srep37526
Netrin-1-Induced Stem Cell Bioactivity Contributes to the Regeneration of Injured Tissues via the Lipid Raft-Dependent Integrin α6β4 Signaling Pathway
Abstract
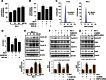
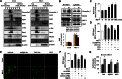
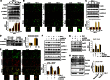
Netrin-1 (Ntn-1) is a multifunctional neuronal signaling molecule; however, its physiological significance, which improves the tissue-regeneration capacity of stem cells, has not been characterized. In the present study, we investigate the mechanism by which Ntn-1 promotes the proliferation of hUCB-MSCs with regard to the regeneration of injured tissues. We found that Ntn-1 induces the proliferation of hUCB-MSCs mainly via Inα6β4 coupled with c-Src. Ntn-1 induced the recruitment of NADPH oxidases and Rac1 into membrane lipid rafts to facilitate ROS production. The Inα6β4 signaling of Ntn-1 through ROS production is uniquely mediated by the activation of SP1 for cell cycle progression and the transcriptional occupancy of SP1 on the VEGF promoter. Moreover, Ntn-1 has the ability to induce the F-actin reorganization of hUCB-MSCs via the Inα6β4 signaling pathway. In an in vivo model, transplantation of hUCB-MSCs pre-treated with Ntn-1 enhanced the skin wound healing process, where relatively more angiogenesis was detected. The potential effect of Ntn-1 on angiogenesis is further verified by the mouse hindlimb ischemia model, where the pre-activation of hUCB-MSCs with Ntn-1 significantly improved vascular regeneration. These results demonstrate that Ntn-1 plays an important role in the tissue regeneration process of hUCB-MSC via the lipid raft-mediated Inα6β4 signaling pathway.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Braun K. M. & Prowse D. M. Distinct epidermal stem cell compartments are maintained by independent niche microenvironments. Stem Cell Rev 2, 221–231 (2006). - PubMed
-
- Yau W. W. Y., Rujitanaroj P. O., Lam L. & Chew S. Y. Directing stem cell fate by controlled RNA interference. Biomaterials 33, 2608–2628 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

