Retinal safety of intravitreal rtPA in healthy rats and under excitotoxic conditions
- PMID: 27881907
- PMCID: PMC5108461
Retinal safety of intravitreal rtPA in healthy rats and under excitotoxic conditions
Abstract
Purpose: Intravitreal recombinant tissue plasminogen activator (rtPA) is used off-label for the surgical management of submacular hemorrhage, a severe complication of neovascular age-related macular degeneration. rtPA is approved for coronary and cerebral thrombolysis. However, in ischemic stroke rtPA is known to increase excitotoxic neural cell death by interacting with the N-methyl-D-aspartate (NMDA) receptor. We therefore investigated the retinal toxicity of rtPA in healthy rats and in a model of NMDA-induced retinal excitotoxicity.
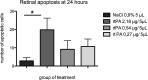
Methods: First, rtPA at three different doses (2.16 µg/5 µl, 0.54 µg/5 µl, and 0.27 µg/5 µl) or vehicle (NaCl 0.9%) was injected intravitreally in healthy rat eyes. Electroretinograms (ERGs) were performed at 24 h or 7 days. Annexin V-fluorescein isothiocyanate (FITC)-labeled apoptotic retinal ganglion cells (RGCs) were counted on flatmounted retinas at 24 h or 7 days. Next, NMDA + vehicle or NMDA + rtPA (0.27 µg/5 µl) was injected intravitreally to generate excitotoxic conditions. Apoptotic annexin V-FITC-labeled RGCs and surviving Brn3a-labeled RGCs were quantified on flatmounted retinas and radial sections, 18 h after treatment.
Results: In healthy rat eyes, the number of apoptotic RGCs was statistically significantly increased 24 h after the administration of rtPA at the highest dose (2.16 µg/5 µl; p = 0.0250) but not at the lower doses of 0.54 and 0.27 µg/5 µl (p = 0.36 and p = 0.20), compared to vehicle. At day 7, there was no difference in the apoptotic RGC count between the rtPA- and vehicle-injected eyes (p = 0.70, p = 0.52, p = 0.11). ERG amplitudes and implicit times were not modified at 24 h or 7 days after injection of any tested rtPA doses, compared to the baseline. Intravitreal administration of NMDA induced RGC death, but under these excitotoxic conditions, coadministration of rtPA did not increase the number of dead RGCs (p = 0.70). Similarly, the number of surviving RGCs on the flatmounted retinas and retinal sections did not differ between the eyes injected with NMDA + vehicle and NMDA + rtPA (p = 0.59 and p = 0.67).
Conclusions: At low clinical equivalent doses corresponding to 25 µg/0.1 ml in humans, intravitreal rtPA is not toxic for healthy rat retinas and does not enhance NMDA-induced excitotoxicity. Vitreal equivalent doses ≥200 µg/0.1 ml should be avoided in patients, due to potential RGC toxicity.
Figures



Similar articles
-
Neuroprotective Effect of Magnesium Acetyltaurate Against NMDA-Induced Excitotoxicity in Rat Retina.Neurotox Res. 2017 Jan;31(1):31-45. doi: 10.1007/s12640-016-9658-9. Epub 2016 Aug 27. Neurotox Res. 2017. PMID: 27568334
-
p53 regulates apoptotic retinal ganglion cell death induced by N-methyl-D-aspartate.Mol Vis. 2002 Sep 15;8:341-50. Mol Vis. 2002. PMID: 12355059
-
The action of 7,8-dihydroxyflavone preserves retinal ganglion cell survival and visual function via the TrkB pathway in NMDA-induced retinal excitotoxicity.Biomed Pharmacother. 2025 Apr;185:117944. doi: 10.1016/j.biopha.2025.117944. Epub 2025 Mar 8. Biomed Pharmacother. 2025. PMID: 40056826
-
Melanopsin+RGCs Are fully Resistant to NMDA-Induced Excitotoxicity.Int J Mol Sci. 2019 Jun 20;20(12):3012. doi: 10.3390/ijms20123012. Int J Mol Sci. 2019. PMID: 31226772 Free PMC article.
-
Literature review of recombinant tissue plasminogen activator used for recent-onset submacular hemorrhage displacement in age-related macular degeneration.Ophthalmologica. 2013;229(1):1-14. doi: 10.1159/000343066. Epub 2012 Oct 12. Ophthalmologica. 2013. PMID: 23075629 Review.
Cited by
-
Retinal Cell Protection in Ocular Excitotoxicity Diseases. Possible Alternatives Offered by Microparticulate Drug Delivery Systems and Future Prospects.Pharmaceutics. 2020 Jan 24;12(2):94. doi: 10.3390/pharmaceutics12020094. Pharmaceutics. 2020. PMID: 31991667 Free PMC article. Review.
References
-
- Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999;213:97–102. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&li... - PubMed
-
- Kiernan DF, Hariprasad SM, Rusu IM, Mehta SV, Mieler WF, Jager RD. Epidemiology of the association between anticoagulants and intraocular hemorrhage in patients with neovascular age-related macular degeneration. Retina. 2010;30:1573–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&li... - PubMed
-
- Toth CA, Morse LS, Hjelmeland LM, Landers MB., 3rd Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol. 1991;109:723–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&li... - PubMed
-
- Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 1982;94:762–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&li... - PubMed
-
- Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257:2912–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&li... - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

