Design and Synthesis of Tesirine, a Clinical Antibody-Drug Conjugate Pyrrolobenzodiazepine Dimer Payload
- PMID: 27882195
- PMCID: PMC5108040
- DOI: 10.1021/acsmedchemlett.6b00062
Design and Synthesis of Tesirine, a Clinical Antibody-Drug Conjugate Pyrrolobenzodiazepine Dimer Payload
Abstract
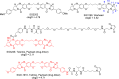
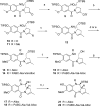
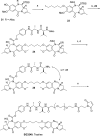
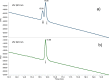
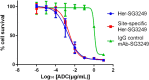
Pyrrolobenzodiazepine dimers are an emerging class of warhead in the field of antibody-drug conjugates (ADCs). Tesirine (SG3249) was designed to combine potent antitumor activity with desirable physicochemical properties such as favorable hydrophobicity and improved conjugation characteristics. One of the reactive imines was capped with a cathepsin B-cleavable valine-alanine linker. A robust synthetic route was developed to allow the production of tesirine on clinical scale, employing a flexible, convergent strategy. Tesirine was evaluated in vitro both in stochastic and engineered ADC constructs and was confirmed as a potent and versatile payload. The conjugation of tesirine to anti-DLL3 rovalpituzumab has resulted in rovalpituzumab-tesirine (Rova-T), currently under evaluation for the treatment of small cell lung cancer.
Keywords: Rova-T; SG3249; Tesirine; antibody−drug conjugates; talirine.
Conflict of interest statement
The authors declare the following competing financial interest(s): A.T., L.M., N.P., L.A., D.W., J.H., and P.H. are Spirogen employees. J.L. is a Stemcentrx employee.
Figures

References
-
- Kung Sutherland M. S.; Walter R. B.; Jeffrey S. C.; Burke P. J.; Yu C.; Kostner H.; Stone I.; Ryan M. C.; Sussman D.; Lyon R. P.; Zeng W.; Harrington K. H.; Klussman K.; Westendorf L.; Meyer D.; Bernstein I. D.; Senter P. D.; Benjamin D. R.; Drachman J. G.; McEarchern J. A. SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 2013, 122 (8), 1455–63. 10.1182/blood-2013-03-491506. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

