Metabolic and adaptive immune responses induced in mice infected with tissue-dwelling nematode Trichinella zimbabwensis
- PMID: 27882304
- PMCID: PMC5116437
- DOI: 10.4314/ovj.v6i3.6
Metabolic and adaptive immune responses induced in mice infected with tissue-dwelling nematode Trichinella zimbabwensis
Abstract
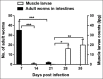
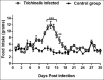
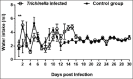
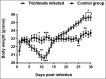
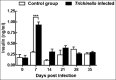
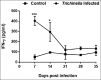
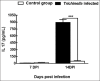
Tissue-dwelling helminths are known to induce intestinal and systemic inflammation accompanied with host compensatory mechanisms to counter balance nutritional and metabolic deficiencies. The metabolic and immune responses of the host depend on parasite species and tissues affected by the parasite. This study investigated metabolic and immuno-inflammatory responses of mice infected with tissue-dwelling larvae of Trichinella zimbabwensis and explored the relationship between infection, metabolic parameters and Th1/Th17 immune responses. Sixty (60) female BALB/c mice aged between 6 to 8 weeks old were randomly assigned into T. zimbabwensis-infected and control groups. Levels of Th1 (interferon-γ) and Th17 (interleukin-17) cytokines, insulin and blood glucose were determined as well as measurements of body weight, food and water intake. Results showed that during the enteric phase of infection, insulin and IFN-γ levels were significantly higher in the Trichinella infected group accompanied with a reduction in the trends of food intake and weight loss compared with the control group. During systemic larval migration, trends in food and water intake were significantly altered and this was attributed to compensatory feeding resulting in weight gain, reduced insulin levels and increased IL-17 levels. Larval migration also induced a Th1/Th17 derived inflammatory response. It was concluded that T. zimbabwensis alters metabolic parameters by instigating host compensatory feeding. Furthermore, we showed for the first time that non-encapsulated T. zimbabwensis parasite plays a role in immunomodulating host Th1/Th17 type responses during chronic infection.
Keywords: Enteric phase; Insulin; Larval migration; Th1 and Th17; Trichinella zimbabwensis.
Figures
Similar articles
-
Metabolomics (Non-Targeted) of Induced Type 2 Diabetic Sprague Dawley Rats Comorbid with a Tissue-Dwelling Nematode Parasite.Int J Mol Sci. 2023 Dec 7;24(24):17211. doi: 10.3390/ijms242417211. Int J Mol Sci. 2023. PMID: 38139040 Free PMC article.
-
Blood glucose, insulin and glycogen profiles in Sprague-Dawley rats co-infected with Plasmodium berghei ANKA and Trichinella zimbabwensis.PeerJ. 2022 Jul 29;10:e13713. doi: 10.7717/peerj.13713. eCollection 2022. PeerJ. 2022. PMID: 35923890 Free PMC article.
-
Differential immune responses in mice infected with the tissue-dwelling nematode Trichinella zimbabwensis.J Helminthol. 2016 Sep;90(5):547-54. doi: 10.1017/S0022149X15000723. Epub 2015 Aug 21. J Helminthol. 2016. PMID: 26294082
-
Trichinella inflammatory myopathy: host or parasite strategy?Parasit Vectors. 2011 Mar 23;4:42. doi: 10.1186/1756-3305-4-42. Parasit Vectors. 2011. PMID: 21429196 Free PMC article. Review.
-
[Methods and tools for parasite differentiation within the genus Trichinella].Wiad Parazytol. 2006;52(3):165-73. Wiad Parazytol. 2006. PMID: 17432239 Review. Polish.
Cited by
-
Cellular immune responses in peripheral blood lymphocytes of Giardia infected squirrel monkey (Saimiri boliviensis boliviensis) treated with Fenbendazole.PLoS One. 2018 Nov 9;13(11):e0198497. doi: 10.1371/journal.pone.0198497. eCollection 2018. PLoS One. 2018. PMID: 30412580 Free PMC article.
-
Trichinella Infection Ameliorated Vincristine-Induced Neuroinflammation in Mice.Korean J Parasitol. 2022 Aug;60(4):247-254. doi: 10.3347/kjp.2022.60.4.247. Epub 2022 Aug 24. Korean J Parasitol. 2022. PMID: 36041486 Free PMC article.
-
Metabolomics (Non-Targeted) of Induced Type 2 Diabetic Sprague Dawley Rats Comorbid with a Tissue-Dwelling Nematode Parasite.Int J Mol Sci. 2023 Dec 7;24(24):17211. doi: 10.3390/ijms242417211. Int J Mol Sci. 2023. PMID: 38139040 Free PMC article.
-
Chemokine, cytokine and haematological profiles in Sprague-Dawley rats co-infected with Plasmodium berghei ANKA and Trichinella zimbabwensis-A laboratory animal model for malaria and tissue-dwelling nematodes co-infection.Heliyon. 2020 Feb 25;6(2):e03475. doi: 10.1016/j.heliyon.2020.e03475. eCollection 2020 Feb. Heliyon. 2020. PMID: 32140591 Free PMC article.
-
Blood glucose, insulin and glycogen profiles in Sprague-Dawley rats co-infected with Plasmodium berghei ANKA and Trichinella zimbabwensis.PeerJ. 2022 Jul 29;10:e13713. doi: 10.7717/peerj.13713. eCollection 2022. PeerJ. 2022. PMID: 35923890 Free PMC article.
References
-
- Adisakwattana P, Nuamtanong S, Kusolsuk T, Chairoj M, Yenchitsomanas P.-T, Chaisri U. Non-encapsulated Trichinella spp T. papuae diminishes severity of DSS-induced colitis in mice. Asian Pac. J. Allergy Immunol. 2013;31:106–114. - PubMed
-
- Appleton J.A, Kennedy M, Harnett W. New insights into the intestinal niche of Trichinella spiralis. Parasitic Nematodes: Molecular Biology, Biochemistry and Immunology. 2001:103–120.
-
- Aranzamendi C, de Bruin A, Kuiper R, Boog C.J.P, van Eden W, Rutten V, Pinelli E. Protection against allergic airway inflammation during the chronic and acute phases of Trichinella spiralis infection. Clin. Exp. Allergy. 2013;43:103–115. - PubMed
-
- Bettelli E, Carrier Y, Gao W, Korn T, Strom T.B, Oukka M, Weiner H.L, Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
-
- Bruschi F, Chiumiento L. Trichinella inflammatory myopathy: host or parasite strategy? Parasit. 2011;Vectors 4:42. http://doi.org/10.1186/1756-3305-4-42 . - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
