Crosstalk between Innate Lymphoid Cells and Other Immune Cells in the Tumor Microenvironment
- PMID: 27882334
- PMCID: PMC5110869
- DOI: 10.1155/2016/7803091
Crosstalk between Innate Lymphoid Cells and Other Immune Cells in the Tumor Microenvironment
Abstract
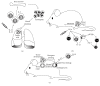
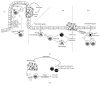
Our knowledge and understanding of the tumor microenvironment (TME) have been recently expanded with the recognition of the important role of innate lymphoid cells (ILC). Three different groups of ILC have been described based on their ability to produce cytokines that mediate the interactions between innate and adaptive immune cells in a variety of immune responses in infection, allergy, and autoimmunity. However, recent evidence from experimental models and clinical studies has demonstrated that ILC contribute to the mechanisms that generate suppressive or tolerant environments that allow tumor regression or progression. Defining the complex network of interactions and crosstalk of ILC with other immune cells and understanding the specific contributions of each type of ILC leading to tumor development will allow the manipulation of their function and will be important to develop new interventions and therapeutic strategies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
The Interplay Between Innate Lymphoid Cells and the Tumor Microenvironment.Front Immunol. 2019 Dec 13;10:2895. doi: 10.3389/fimmu.2019.02895. eCollection 2019. Front Immunol. 2019. PMID: 31921156 Free PMC article. Review.
-
Innate lymphoid cells as regulators of the tumor microenvironment.Semin Immunol. 2019 Feb;41:101270. doi: 10.1016/j.smim.2019.03.002. Epub 2019 Mar 11. Semin Immunol. 2019. PMID: 30871769 Review.
-
The Yin and Yang of Innate Lymphoid Cells in Cancer.Immunol Lett. 2016 Nov;179:29-35. doi: 10.1016/j.imlet.2016.06.003. Epub 2016 Jun 11. Immunol Lett. 2016. PMID: 27296768 Review.
-
Innate lymphoid cells and cancer: Role in tumor progression and inhibition.Eur J Immunol. 2021 Sep;51(9):2188-2205. doi: 10.1002/eji.202049033. Epub 2021 Jul 11. Eur J Immunol. 2021. PMID: 34189723 Free PMC article. Review.
-
Innate Lymphoid Cells in Protection, Pathology, and Adaptive Immunity During Apicomplexan Infection.Front Immunol. 2019 Feb 28;10:196. doi: 10.3389/fimmu.2019.00196. eCollection 2019. Front Immunol. 2019. PMID: 30873151 Free PMC article. Review.
Cited by
-
Communication Pattern Changes Along With Declined IGF1 of Immune Cells in COVID-19 Patients During Disease Progression.Front Immunol. 2022 Jan 14;12:729990. doi: 10.3389/fimmu.2021.729990. eCollection 2021. Front Immunol. 2022. PMID: 35095832 Free PMC article.
-
The role of innate lymphoid cells in response to microbes at mucosal surfaces.Mucosal Immunol. 2020 May;13(3):399-412. doi: 10.1038/s41385-020-0265-y. Epub 2020 Feb 11. Mucosal Immunol. 2020. PMID: 32047273 Free PMC article. Review.
-
Current challenges for cancer vaccine adjuvant development.Expert Rev Vaccines. 2018 Mar;17(3):207-215. doi: 10.1080/14760584.2018.1434000. Epub 2018 Feb 8. Expert Rev Vaccines. 2018. PMID: 29372660 Free PMC article. Review.
-
Innate Lymphoid Cells: Regulators of Gut Barrier Function and Immune Homeostasis.J Immunol Res. 2019 Dec 20;2019:2525984. doi: 10.1155/2019/2525984. eCollection 2019. J Immunol Res. 2019. PMID: 31930146 Free PMC article. Review.
-
Microbial metabolites are involved in tumorigenesis and development by regulating immune responses.Front Immunol. 2023 Dec 19;14:1290414. doi: 10.3389/fimmu.2023.1290414. eCollection 2023. Front Immunol. 2023. PMID: 38169949 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

