ML372 blocks SMN ubiquitination and improves spinal muscular atrophy pathology in mice
- PMID: 27882347
- PMCID: PMC5111506
- DOI: 10.1172/jci.insight.88427
ML372 blocks SMN ubiquitination and improves spinal muscular atrophy pathology in mice
Abstract
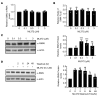
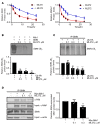
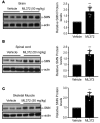
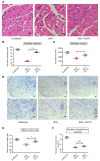
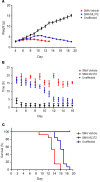
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disease and one of the leading inherited causes of infant mortality. SMA results from insufficient levels of the survival motor neuron (SMN) protein, and studies in animal models of the disease have shown that increasing SMN protein levels ameliorates the disease phenotype. Our group previously identified and optimized a new series of small molecules, with good potency and toxicity profiles and reasonable pharmacokinetics, that were able to increase SMN protein levels in SMA patient-derived cells. We show here that ML372, a representative of this series, almost doubles the half-life of residual SMN protein expressed from the SMN2 locus by blocking its ubiquitination and subsequent degradation by the proteasome. ML372 increased SMN protein levels in muscle, spinal cord, and brain tissue of SMA mice. Importantly, ML372 treatment improved the righting reflex and extended survival of a severe mouse model of SMA. These results demonstrate that slowing SMN degradation by selectively inhibiting its ubiquitination can improve the motor phenotype and lifespan of SMA model mice.
Conflict of interest statement
The authors declare that no conflict of interest exists.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

