Repression of RNA polymerase by the archaeo-viral regulator ORF145/RIP
- PMID: 27882920
- PMCID: PMC5123050
- DOI: 10.1038/ncomms13595
Repression of RNA polymerase by the archaeo-viral regulator ORF145/RIP
Abstract
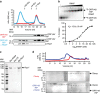
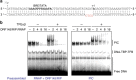
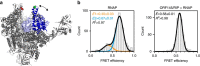
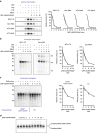
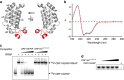
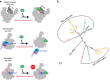
Little is known about how archaeal viruses perturb the transcription machinery of their hosts. Here we provide the first example of an archaeo-viral transcription factor that directly targets the host RNA polymerase (RNAP) and efficiently represses its activity. ORF145 from the temperate Acidianus two-tailed virus (ATV) forms a high-affinity complex with RNAP by binding inside the DNA-binding channel where it locks the flexible RNAP clamp in one position. This counteracts the formation of transcription pre-initiation complexes in vitro and represses abortive and productive transcription initiation, as well as elongation. Both host and viral promoters are subjected to ORF145 repression. Thus, ORF145 has the properties of a global transcription repressor and its overexpression is toxic for Sulfolobus. On the basis of its properties, we have re-named ORF145 RNAP Inhibitory Protein (RIP).
Figures

Similar articles
-
Structural basis of RNA polymerase inhibition by viral and host factors.Nat Commun. 2021 Sep 17;12(1):5523. doi: 10.1038/s41467-021-25666-5. Nat Commun. 2021. PMID: 34535646 Free PMC article.
-
Activation and repression of transcription at two different phage phi29 promoters are mediated by interaction of the same residues of regulatory protein p4 with RNA polymerase.EMBO J. 1996 Jan 15;15(2):383-91. EMBO J. 1996. PMID: 8617213 Free PMC article.
-
Correlating Transcription Initiation and Conformational Changes by a Single-Subunit RNA Polymerase with Near Base-Pair Resolution.Mol Cell. 2018 May 17;70(4):695-706.e5. doi: 10.1016/j.molcel.2018.04.018. Mol Cell. 2018. PMID: 29775583 Free PMC article.
-
How to Shut Down Transcription in Archaea during Virus Infection.Microorganisms. 2022 Sep 13;10(9):1824. doi: 10.3390/microorganisms10091824. Microorganisms. 2022. PMID: 36144426 Free PMC article. Review.
-
Archaeology of RNA polymerase: factor swapping during the transcription cycle.Biochem Soc Trans. 2013 Feb 1;41(1):362-7. doi: 10.1042/BST20120274. Biochem Soc Trans. 2013. PMID: 23356312 Review.
Cited by
-
The First MS-Cleavable, Photo-Thiol-Reactive Cross-Linker for Protein Structural Studies.J Am Soc Mass Spectrom. 2019 Jan;30(1):139-148. doi: 10.1007/s13361-018-1952-8. Epub 2018 Apr 20. J Am Soc Mass Spectrom. 2019. PMID: 29679287
-
TFS and Spt4/5 accelerate transcription through archaeal histone-based chromatin.Mol Microbiol. 2019 Mar;111(3):784-797. doi: 10.1111/mmi.14191. Epub 2019 Feb 1. Mol Microbiol. 2019. PMID: 30592095 Free PMC article.
-
Multisubunit DNA-Dependent RNA Polymerases from Vaccinia Virus and Other Nucleocytoplasmic Large-DNA Viruses: Impressions from the Age of Structure.Microbiol Mol Biol Rev. 2017 Jul 12;81(3):e00010-17. doi: 10.1128/MMBR.00010-17. Print 2017 Sep. Microbiol Mol Biol Rev. 2017. PMID: 28701329 Free PMC article. Review.
-
The biology of thermoacidophilic archaea from the order Sulfolobales.FEMS Microbiol Rev. 2021 Aug 17;45(4):fuaa063. doi: 10.1093/femsre/fuaa063. FEMS Microbiol Rev. 2021. PMID: 33476388 Free PMC article.
-
ORF4 of the Temperate Archaeal Virus SNJ1 Governs the Lysis-Lysogeny Switch and Superinfection Immunity.J Virol. 2020 Jul 30;94(16):e00841-20. doi: 10.1128/JVI.00841-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32522850 Free PMC article.
References
-
- Gottesman M. Bacteriophage lambda: the untold story. J. Mol. Biol. 293, 177–180 (1999). - PubMed
-
- Forterre P. & Prangishvili D. The major role of viruses in cellular evolution: facts and hypotheses. Curr. Opin. Virol. 3, 558–565 (2013). - PubMed
-
- Prangishvili D., Forterre P. & Garrett R. A. Viruses of the Archaea: a unifying view. Nat. Rev. Microbiol. 4, 837–848 (2006). - PubMed
-
- Ortmann A. C., Wiedenheft B., Douglas T. & Young M. Hot crenarchaeal viruses reveal deep evolutionary connections. Nat. Rev. Microbiol. 4, 520–528 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

