A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices
- PMID: 27883002
- PMCID: PMC5122307
- DOI: 10.1038/npjpcrm.2016.79
A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices
Erratum in
-
A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: comparison of ELLIPTA with other inhaler devices.NPJ Prim Care Respir Med. 2017 Mar 23;27:17001. doi: 10.1038/npjpcrm.2017.1. NPJ Prim Care Respir Med. 2017. PMID: 28333128 Free PMC article. No abstract available.
Abstract
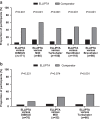
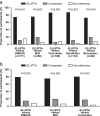
Errors in the use of different inhalers were investigated in patients naive to the devices under investigation in a multicentre, single-visit, randomised, open-label, cross-over study. Patients with chronic obstructive pulmonary disease (COPD) or asthma were assigned to ELLIPTA vs DISKUS (Accuhaler), metered-dose inhaler (MDI) or Turbuhaler. Patients with COPD were also assigned to ELLIPTA vs Handihaler or Breezhaler. Patients demonstrated inhaler use after reading the patient information leaflet (PIL). A trained investigator assessed critical errors (i.e., those likely to result in the inhalation of significantly reduced, minimal or no medication). If the patient made errors, the investigator demonstrated the correct use of the inhaler, and the patient demonstrated inhaler use again. Fewer COPD patients made critical errors with ELLIPTA after reading the PIL vs: DISKUS, 9/171 (5%) vs 75/171 (44%); MDI, 10/80 (13%) vs 48/80 (60%); Turbuhaler, 8/100 (8%) vs 44/100 (44%); Handihaler, 17/118 (14%) vs 57/118 (48%); Breezhaler, 13/98 (13%) vs 45/98 (46%; all P<0.001). Most patients (57-70%) made no errors using ELLIPTA and did not require investigator instruction. Instruction was required for DISKUS (65%), MDI (85%), Turbuhaler (71%), Handihaler (62%) and Breezhaler (56%). Fewer asthma patients made critical errors with ELLIPTA after reading the PIL vs: DISKUS (3/70 (4%) vs 9/70 (13%), P=0.221); MDI (2/32 (6%) vs 8/32 (25%), P=0.074) and significantly fewer vs Turbuhaler (3/60 (5%) vs 20/60 (33%), P<0.001). More asthma and COPD patients preferred ELLIPTA over the other devices (all P⩽0.002). Significantly, fewer COPD patients using ELLIPTA made critical errors after reading the PIL vs other inhalers. More asthma and COPD patients preferred ELLIPTA over comparator inhalers.
Trial registration: ClinicalTrials.gov NCT02184624 NCT02195284.
Conflict of interest statement
JvdP has no shares in any pharmaceutical companies. He has received sponsorship to carry out studies and has participated in Advisory Boards from several pharmaceutical companies that market inhaled products. These include Almirall, Boehringer Ingelheim (BI), GlaxoSmithKline (GSK), Mundipharma and Teva. Neither MT nor any member of his close family has any shares in pharmaceutical companies. In the past 3 years, he has received speaker’s honoraria for speaking at sponsored meetings or satellite symposia at conferences from the following companies marketing respiratory and allergy products: Aerocrine, AstraZeneca, BI, GSK, Teva and Novartis. He has received honoraria for attending advisory panels with: Aerocrine, Almirall, AstraZeneca, BI, Chiesi, GSK, MSD, Novartis, Pfizer, Roche and Sandoz. He has received sponsorship to attend international scientific meetings from: GSK and AstraZeneca. He has received funding for research projects from GSK. He is a member of the BTS SIGN Asthma guideline group and the NICE Asthma guideline group. HC has no shares in any pharmaceutical companies. He has received sponsorship from several pharmaceutical companies that market inhaled products to carry out: clinical studies, Board Membership, consultant agreements and honoraria for presentations. These include Almirall, AstraZeneca, BI, Chiesi, GSK, Innovata Biomed, Meda, Napp Pharmaceuticals, Mundipharma, NorPharma, Norvartis, Orion, Sanofi, Teva, Truddell Medical International, UCB and Zentiva. Research sponsorship has also been received from grant awarding bodies (EPSRC and MRC). He is the owner of Inhalation Consultancy Ltd and a director of Talmedica Ltd. TW has received research funding and consultancy fees from GSK, AstraZeneca and BI. He is a Director of my mHealth Ltd. CS has no shares in any pharmaceutical companies. She has received consultant agreements and honoraria for presentations from several pharmaceutical companies that market inhaled medication. These include AstraZeneca, Chiesi, GSK, Napp Pharmaceuticals and Teva. NB, RS, VI, HS and C-QZ are employees of GSK and hold stock in GSK. The remaining authors declare no conflict of interest.
Figures
References
-
- Virchow, J. C. et al. Importance of inhaler devices in the management of airway disease. Respir. Med. 102, 10–17 (2008). - PubMed
-
- Bousquet, J. et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J. Allergy Clin. Immunol. 126, 926–938 (2010). - PubMed
-
- Giraud, V. & Roche, N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur. Respir. J. 19, 246–251 (2002). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical