Telomeres are elongated in older individuals in a hibernating rodent, the edible dormouse (Glis glis)
- PMID: 27883035
- PMCID: PMC5121655
- DOI: 10.1038/srep36856
Telomeres are elongated in older individuals in a hibernating rodent, the edible dormouse (Glis glis)
Abstract
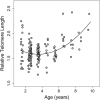
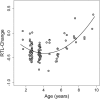
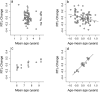
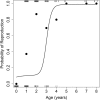
Telomere shortening is thought to be an important biomarker for life history traits such as lifespan and aging, and can be indicative of genome integrity, survival probability and the risk of cancer development. In humans and other animals, telomeres almost always shorten with age, with more rapid telomere attrition in short-lived species. Here, we show that in the edible dormouse (Glis glis) telomere length significantly increases from an age of 6 to an age of 9 years. While this finding could be due to higher survival of individuals with longer telomeres, we also found, using longitudinal measurements, a positive effect of age on the rate of telomere elongation within older individuals. To our knowledge, no previous study has reported such an effect of age on telomere lengthening. We attribute this exceptional pattern to the peculiar life-history of this species, which skips reproduction in years with low food availability. Further, we show that this "sit tight" strategy in the timing of reproduction is associated with an increasing likelihood for an individual to reproduce as it ages. As reproduction could facilitate telomere attrition, this life-history strategy may have led to the evolution of increased somatic maintenance and telomere elongation with increasing age.
Figures
Similar articles
-
Seasonal variation in telomere length of a hibernating rodent.Biol Lett. 2013 Feb 6;9(2):20121095. doi: 10.1098/rsbl.2012.1095. Print 2013 Apr 23. Biol Lett. 2013. PMID: 23389666 Free PMC article.
-
Telomere dynamics in free-living edible dormice (Glis glis): the impact of hibernation and food supply.J Exp Biol. 2016 Aug 15;219(Pt 16):2469-74. doi: 10.1242/jeb.140871. J Exp Biol. 2016. PMID: 27535986 Free PMC article.
-
Telomere dynamics in a long-lived bird, the barnacle goose.BMC Evol Biol. 2012 Dec 31;12:257. doi: 10.1186/1471-2148-12-257. BMC Evol Biol. 2012. PMID: 23273548 Free PMC article.
-
Does Reproduction Shorten Telomeres? Towards Integrating Individual Quality with Life-History Strategies in Telomere Biology.Bioessays. 2019 Nov;41(11):e1900095. doi: 10.1002/bies.201900095. Epub 2019 Oct 2. Bioessays. 2019. PMID: 31577044 Review.
-
Understanding seasonal telomere length dynamics in hibernating species.J Therm Biol. 2024 Jul;123:103913. doi: 10.1016/j.jtherbio.2024.103913. Epub 2024 Jul 5. J Therm Biol. 2024. PMID: 39002254 Review.
Cited by
-
Impact of trainability on telomere dynamics of pet dogs (Canis lupus familiaris): An explorative study in aging dogs.PLoS One. 2025 Feb 5;20(2):e0317332. doi: 10.1371/journal.pone.0317332. eCollection 2025. PLoS One. 2025. PMID: 39908316 Free PMC article.
-
Evidence of Paternal Effects on Telomere Length Increases in Early Life.Front Genet. 2022 May 17;13:880455. doi: 10.3389/fgene.2022.880455. eCollection 2022. Front Genet. 2022. PMID: 35656320 Free PMC article.
-
Effects of aging on timing of hibernation and reproduction.Sci Rep. 2018 Sep 17;8(1):13881. doi: 10.1038/s41598-018-32311-7. Sci Rep. 2018. PMID: 30224823 Free PMC article.
-
Food availability positively affects the survival and somatic maintenance of hibernating garden dormice (Eliomys quercinus).Front Zool. 2023 May 24;20(1):19. doi: 10.1186/s12983-023-00498-9. Front Zool. 2023. PMID: 37226260 Free PMC article.
-
Different effects of accelerated development and enhanced growth on oxidative stress and telomere shortening in amphibian larvae.Sci Rep. 2017 Aug 8;7(1):7494. doi: 10.1038/s41598-017-07201-z. Sci Rep. 2017. PMID: 28790317 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

