Structural and functional probing of PorZ, an essential bacterial surface component of the type-IX secretion system of human oral-microbiomic Porphyromonas gingivalis
- PMID: 27883039
- PMCID: PMC5121618
- DOI: 10.1038/srep37708
Structural and functional probing of PorZ, an essential bacterial surface component of the type-IX secretion system of human oral-microbiomic Porphyromonas gingivalis
Abstract
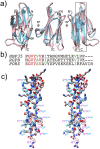
Porphyromonas gingivalis is a member of the human oral microbiome abundant in dysbiosis and implicated in the pathogenesis of periodontal (gum) disease. It employs a newly described type-IX secretion system (T9SS) for secretion of virulence factors. Cargo proteins destined for secretion through T9SS carry a recognition signal in the conserved C-terminal domain (CTD), which is removed by sortase PorU during translocation. Here, we identified a novel component of T9SS, PorZ, which is essential for surface exposure of PorU and posttranslational modification of T9SS cargo proteins. These include maturation of enzyme precursors, CTD removal and attachment of anionic lipopolysaccharide for anchorage in the outer membrane. The crystal structure of PorZ revealed two β-propeller domains and a C-terminal β-sandwich domain, which conforms to the canonical CTD architecture. We further documented that PorZ is itself transported to the cell surface via T9SS as a full-length protein with its CTD intact, independently of the presence or activity of PorU. Taken together, our results shed light on the architecture and possible function of a novel component of the T9SS. Knowledge of how T9SS operates will contribute to our understanding of protein secretion as part of host-microbiome interactions by dysbiotic members of the human oral cavity.
Figures
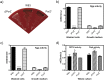
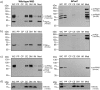
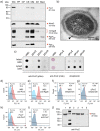
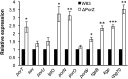

Similar articles
-
PorZ, an Essential Component of the Type IX Secretion System of Porphyromonas gingivalis, Delivers Anionic Lipopolysaccharide to the PorU Sortase for Transpeptidase Processing of T9SS Cargo Proteins.mBio. 2021 Feb 23;12(1):e02262-20. doi: 10.1128/mBio.02262-20. mBio. 2021. PMID: 33622730 Free PMC article.
-
Intermolecular latency regulates the essential C-terminal signal peptidase and sortase of the Porphyromonas gingivalis type-IX secretion system.Proc Natl Acad Sci U S A. 2021 Oct 5;118(40):e2103573118. doi: 10.1073/pnas.2103573118. Epub 2021 Sep 30. Proc Natl Acad Sci U S A. 2021. PMID: 34593635 Free PMC article.
-
PG1058 Is a Novel Multidomain Protein Component of the Bacterial Type IX Secretion System.PLoS One. 2016 Oct 6;11(10):e0164313. doi: 10.1371/journal.pone.0164313. eCollection 2016. PLoS One. 2016. PMID: 27711252 Free PMC article.
-
Type IX secretion: the generation of bacterial cell surface coatings involved in virulence, gliding motility and the degradation of complex biopolymers.Mol Microbiol. 2017 Oct;106(1):35-53. doi: 10.1111/mmi.13752. Epub 2017 Aug 9. Mol Microbiol. 2017. PMID: 28714554 Review.
-
The Type IX Secretion System (T9SS): Highlights and Recent Insights into Its Structure and Function.Front Cell Infect Microbiol. 2017 May 26;7:215. doi: 10.3389/fcimb.2017.00215. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28603700 Free PMC article. Review.
Cited by
-
Gingimaps: Protein Localization in the Oral Pathogen Porphyromonas gingivalis.Microbiol Mol Biol Rev. 2020 Jan 2;84(1):e00032-19. doi: 10.1128/MMBR.00032-19. Print 2020 Feb 19. Microbiol Mol Biol Rev. 2020. PMID: 31896547 Free PMC article. Review.
-
Structural insights unravel the zymogenic mechanism of the virulence factor gingipain K from Porphyromonas gingivalis, a causative agent of gum disease from the human oral microbiome.J Biol Chem. 2017 Apr 7;292(14):5724-5735. doi: 10.1074/jbc.M117.776724. Epub 2017 Feb 14. J Biol Chem. 2017. PMID: 28196869 Free PMC article.
-
Phylogenetic comparison between Type IX Secretion System (T9SS) protein components suggests evidence of horizontal gene transfer.PeerJ. 2020 Jun 26;8:e9019. doi: 10.7717/peerj.9019. eCollection 2020. PeerJ. 2020. PMID: 32617187 Free PMC article.
-
Prediction of Selected Biosynthetic Pathways for the Lipopolysaccharide Components in Porphyromonas gingivalis.Pathogens. 2021 Mar 20;10(3):374. doi: 10.3390/pathogens10030374. Pathogens. 2021. PMID: 33804654 Free PMC article.
-
Overexpression and characterization of a novel GH16 β-agarase (Aga1) from Cellulophaga omnivescoria W5C.Biotechnol Lett. 2020 Nov;42(11):2231-2238. doi: 10.1007/s10529-020-02933-x. Epub 2020 Jun 9. Biotechnol Lett. 2020. PMID: 32519168
References
-
- Desvaux M., Hebraud M., Talon R. & Henderson I. R. Outer membrane translocation: numerical protein secretion nomenclature in question in mycobacteria. Trends Microbiol. 17, 338–340 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

