mQC: A Heuristic Quality-Control Metric for High-Throughput Drug Combination Screening
- PMID: 27883049
- PMCID: PMC5121902
- DOI: 10.1038/srep37741
mQC: A Heuristic Quality-Control Metric for High-Throughput Drug Combination Screening
Abstract
Quality control (QC) metrics are critical in high throughput screening (HTS) platforms to ensure reliability and confidence in assay data and downstream analyses. Most reported HTS QC metrics are designed for plate level or single well level analysis. With the advent of high throughput combination screening there is a need for QC metrics that quantify the quality of combination response matrices. We introduce a predictive, interpretable, matrix-level QC metric, mQC, based on a mix of data-derived and heuristic features. mQC accurately reproduces the expert assessment of combination response quality and correctly identifies unreliable response matrices that can lead to erroneous or misleading characterization of synergy. When combined with the plate-level QC metric, Z', mQC provides a more appropriate determination of the quality of a drug combination screen. Retrospective analysis on a number of completed combination screens further shows that mQC is able to identify problematic screens whereas plate-level QC was not able to. In conclusion, our data indicates that mQC is a reliable QC filter that can be used to identify problematic drug combinations matrices and prevent further analysis on erroneously active combinations as well as for troubleshooting failed screens. The R source code of mQC is available at http://matrix.ncats.nih.gov/mQC.
Figures




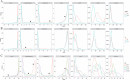
 . (B) Distribution of DBNorm with different fractions of random error (p.random) introduced in the model. (C–E) Distribution of DBNorm based on mQC, Z’ or QC Mott et al. (F–H) Distribution of gamma based on mQC, Z’ or QC Mott et al.
. (B) Distribution of DBNorm with different fractions of random error (p.random) introduced in the model. (C–E) Distribution of DBNorm based on mQC, Z’ or QC Mott et al. (F–H) Distribution of gamma based on mQC, Z’ or QC Mott et al.

References
-
- Malo N., Hanley J., Cerquozzi S., Pelletier J. & Nadon R. Statistical Practice in High-Throughput Screening Data Analysis. Nat.~Biotech. 24, 167–175 (2006). - PubMed
-
- Iversen P. W. et al. In Assay Guidance Manual (eds Sittampalam G. S. et al.) (2004).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

