The Warburg Effect Mediator Pyruvate Kinase M2 Expression and Regulation in the Retina
- PMID: 27883057
- PMCID: PMC5121888
- DOI: 10.1038/srep37727
The Warburg Effect Mediator Pyruvate Kinase M2 Expression and Regulation in the Retina
Abstract
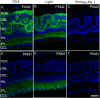
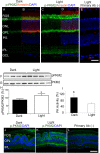
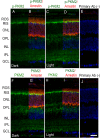
The tumor form of pyruvate kinase M2 (PKM2) undergoes tyrosine phosphorylation and gives rise to the Warburg effect. The Warburg effect defines a pro-oncogenic metabolism switch such that cancer cells take up more glucose than normal tissue and favor incomplete oxidation of glucose, even in the presence of oxygen. Retinal photoreceptors are highly metabolic and their energy consumption is equivalent to that of a multiplying tumor cell. In the present study, we found that PKM2 is the predominant isoform in both rod- and cone-dominant retina, and that it undergoes a light-dependent tyrosine phosphorylation. We also discovered that PKM2 phosphorylation is signaled through photobleaching of rhodopsin. Our findings suggest that phosphoinositide 3-kinase activation promotes PKM2 phosphorylation. Light and tyrosine phosphorylation appear to regulate PKM2 to provide a metabolic advantage to photoreceptor cells, thereby promoting cell survival.
Figures



Similar articles
-
Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth.Sci Signal. 2009 Nov 17;2(97):ra73. doi: 10.1126/scisignal.2000431. Sci Signal. 2009. PMID: 19920251 Free PMC article.
-
Pyruvate kinase M2 isoform deletion in cone photoreceptors results in age-related cone degeneration.Cell Death Dis. 2018 Jul 3;9(7):737. doi: 10.1038/s41419-018-0712-9. Cell Death Dis. 2018. PMID: 29970877 Free PMC article.
-
Pyruvate kinase M2 affects liver cancer cell behavior through up-regulation of HIF-1α and Bcl-xL in culture.Biomed Pharmacother. 2015 Feb;69:277-84. doi: 10.1016/j.biopha.2014.12.010. Epub 2014 Dec 19. Biomed Pharmacother. 2015. PMID: 25661370
-
Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells.Int J Biochem Cell Biol. 2011 Jul;43(7):969-80. doi: 10.1016/j.biocel.2010.02.005. Epub 2010 Feb 13. Int J Biochem Cell Biol. 2011. PMID: 20156581 Review.
-
Cancer-like metabolism of the mammalian retina.Clin Exp Ophthalmol. 2015 May-Jun;43(4):367-76. doi: 10.1111/ceo.12462. Epub 2014 Nov 7. Clin Exp Ophthalmol. 2015. PMID: 25330055 Review.
Cited by
-
Oxidative stress-induced alterations in retinal glucose metabolism in Retinitis Pigmentosa.Free Radic Biol Med. 2022 Mar;181:143-153. doi: 10.1016/j.freeradbiomed.2022.01.032. Epub 2022 Feb 5. Free Radic Biol Med. 2022. PMID: 35134532 Free PMC article.
-
PKM2 ablation enhanced retinal function and survival in a preclinical model of retinitis pigmentosa.Mamm Genome. 2020 Apr;31(3-4):77-85. doi: 10.1007/s00335-020-09837-1. Epub 2020 Apr 27. Mamm Genome. 2020. PMID: 32342224 Free PMC article.
-
Pyruvate kinase M2 regulates photoreceptor structure, function, and viability.Cell Death Dis. 2018 Feb 14;9(2):240. doi: 10.1038/s41419-018-0296-4. Cell Death Dis. 2018. PMID: 29445082 Free PMC article.
-
Age-Related Changes in the Glycolytic Enzymes of M2-Isoform of Pyruvate Kinase and Fructose-1,6-Bisphosphate Aldolase: Implications to Age-Related Macular Degeneration.Aging Dis. 2024 Oct 1;15(5):2271-2283. doi: 10.14336/AD.2024.0077. Aging Dis. 2024. PMID: 38739943 Free PMC article.
-
Development of Novel Small-Molecule Activators of Pyruvate Kinase Muscle Isozyme 2, PKM2, to Reduce Photoreceptor Apoptosis.Pharmaceuticals (Basel). 2023 May 6;16(5):705. doi: 10.3390/ph16050705. Pharmaceuticals (Basel). 2023. PMID: 37242488 Free PMC article.
References
-
- Ng S. K. et al.. Cancer-like metabolism of the mammalian retina. Clin. Experiment. Ophthalmol (2014). - PubMed
-
- Casson R. J., Chidlow G., Han G. & Wood J. P. An explanation for the Warburg effect in the adult mammalian retina. Clin. Experiment. Ophthalmol 41, 517 (2013). - PubMed
-
- Fiske B. P. & Vander Heiden M. G. Seeing the Warburg effect in the developing retina. Nat. Cell Biol 14, 790–791 (2012). - PubMed
-
- Warburg O. On respiratory impairment in cancer cells. Science 124, 269–270 (1956). - PubMed
-
- Warburg O. On the origin of cancer cells. Science 123, 309–314 (1956). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

