Environmental Intervention as a Therapy for Adverse Programming by Ancestral Stress
- PMID: 27883060
- PMCID: PMC5121646
- DOI: 10.1038/srep37814
Environmental Intervention as a Therapy for Adverse Programming by Ancestral Stress
Abstract
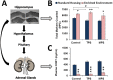
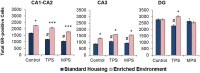
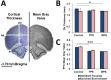
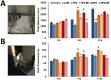
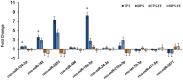
Ancestral stress can program stress sensitivity and health trajectories across multiple generations. While ancestral stress is uncontrollable to the filial generations, it is critical to identify therapies that overcome transgenerational programming. Here we report that prenatal stress in rats generates a transgenerationally heritable endocrine and epigenetic footprint and elevated stress sensitivity which can be alleviated by beneficial experiences in later life. Ancestral stress led to downregulated glucocorticoid receptor and prefrontal cortex neuronal densities along with precocious development of anxiety-like behaviours. Environmental enrichment (EE) during adolescence mitigated endocrine and neuronal markers of stress and improved miR-182 expression linked to brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) regulation in stressed lineages. Thus, EE may serve as a powerful intervention for adverse transgenerational programming through microRNA-mediated regulation of BDNF and NT-3 pathways. The identification of microRNAs that mediate the actions of EE highlights new therapeutic strategies for mental health conditions and psychiatric disease.
Figures

Similar articles
-
Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health.Neurosci Biobehav Rev. 2015 Jan;48:70-91. doi: 10.1016/j.neubiorev.2014.11.013. Epub 2014 Nov 24. Neurosci Biobehav Rev. 2015. PMID: 25464029 Review.
-
Ancestral experience as a game changer in stress vulnerability and disease outcomes.Bioessays. 2015 Jun;37(6):602-11. doi: 10.1002/bies.201400217. Epub 2015 Mar 11. Bioessays. 2015. PMID: 25759985 Review.
-
Active coping of prenatally stressed rats in the forced swimming test: involvement of the Nurr1 gene.Stress. 2016 Sep;19(5):506-15. doi: 10.1080/10253890.2016.1193147. Epub 2016 Jun 13. Stress. 2016. PMID: 27219004
-
Delayed BDNF alterations in the prefrontal cortex of rats exposed to prenatal stress: preventive effect of lurasidone treatment during adolescence.Eur Neuropsychopharmacol. 2014 Jun;24(6):986-95. doi: 10.1016/j.euroneuro.2013.12.010. Epub 2013 Dec 30. Eur Neuropsychopharmacol. 2014. PMID: 24440552
-
Environmental enrichment mitigates the impact of ancestral stress on motor skill and corticospinal tract plasticity.Neurosci Lett. 2016 Oct 6;632:181-6. doi: 10.1016/j.neulet.2016.08.059. Epub 2016 Sep 1. Neurosci Lett. 2016. PMID: 27592512
Cited by
-
Perinatal Psychoneuroimmunology of Prenatal Stress and Its Effects on Fetal and Postnatal Brain Development.Methods Mol Biol. 2025;2868:303-332. doi: 10.1007/978-1-0716-4200-9_16. Methods Mol Biol. 2025. PMID: 39546237
-
Ancestral Stress Alters Lifetime Mental Health Trajectories and Cortical Neuromorphology via Epigenetic Regulation.Sci Rep. 2019 Apr 23;9(1):6389. doi: 10.1038/s41598-019-42691-z. Sci Rep. 2019. PMID: 31011159 Free PMC article.
-
Environmental enrichment reverses cerebellar impairments caused by prenatal exposure to a synthetic glucocorticoid.AIMS Neurosci. 2022 Jul 14;9(3):320-344. doi: 10.3934/Neuroscience.2022018. eCollection 2022. AIMS Neurosci. 2022. PMID: 36329900 Free PMC article.
-
An enriched environment reduces chronic stress-induced visceral pain through modulating microglial activity in the central nucleus of the amygdala.Am J Physiol Gastrointest Liver Physiol. 2022 Feb 1;322(2):G223-G233. doi: 10.1152/ajpgi.00307.2021. Epub 2021 Dec 8. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 34877892 Free PMC article.
-
Critical period regulation across multiple timescales.Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23242-23251. doi: 10.1073/pnas.1820836117. Epub 2020 Jun 5. Proc Natl Acad Sci U S A. 2020. PMID: 32503914 Free PMC article. Review.
References
-
- Gitau R., Fisk N. M. & Glover V. Maternal stress in pregnancy and its effect on the human foetus: an overview of research findings. Stress 4, 195–203 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

