Pretreatment of activated human CD8 T cells with IL-12 leads to enhanced TCR-induced signaling and cytokine production
- PMID: 27883938
- PMCID: PMC5201458
- DOI: 10.1016/j.molimm.2016.11.008
Pretreatment of activated human CD8 T cells with IL-12 leads to enhanced TCR-induced signaling and cytokine production
Abstract
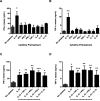
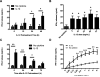
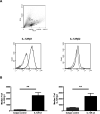
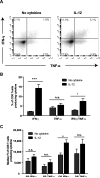
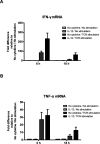
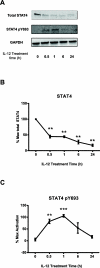
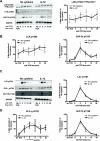
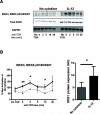
During the immune response to pathogens and autoantigens, CD8T cells are exposed to numerous inflammatory agents including the cytokine IL-12. Previous studies have focused on how IL-12 regulates T cell functions when present during or after the activation of the T cell receptor (TCR). However, recent studies suggest that prior exposure to IL-12 also alters the TCR responsiveness of murine T cells. Whether similar phenomena occur in human activated CD8T cells and the mechanisms mediating these effects remain unexplored. In this study, we observed that pretreatment of human activated CD8T cells with IL-12 results in increased cytokine mRNA and protein production following subsequent TCR challenge. The potentiation of TCR-mediated cytokine release was transient and required low doses of IL-12 for at least 24h. Mechanistically, prior exposure to IL-12 increased the TCR induced activation of select MAPKs and AKT without altering the activation of more proximal TCR signaling molecules, suggesting that the IL-12 mediated changes in TCR signaling are responsible for the increased production of cytokines. Our data suggest that prior treatment with IL-12 potentiates human CD8T cell responses at sites of infection and inflammation, expanding our understanding of the function of this clinically important cytokine.
Keywords: CD8T cells; Human T cells; IL-12; TCR signaling.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells.Eur J Immunol. 2011 Nov;41(11):3351-60. doi: 10.1002/eji.201141629. Epub 2011 Oct 13. Eur J Immunol. 2011. PMID: 21887788 Free PMC article.
-
Exposure of Human CD4 T Cells to IL-12 Results in Enhanced TCR-Induced Cytokine Production, Altered TCR Signaling, and Increased Oxidative Metabolism.PLoS One. 2016 Jun 9;11(6):e0157175. doi: 10.1371/journal.pone.0157175. eCollection 2016. PLoS One. 2016. PMID: 27280403 Free PMC article.
-
Prior TLR5 induction in human T cells results in a transient potentiation of subsequent TCR-induced cytokine production.Mol Immunol. 2014 Feb;57(2):161-70. doi: 10.1016/j.molimm.2013.09.002. Epub 2013 Oct 12. Mol Immunol. 2014. PMID: 24128895 Free PMC article.
-
Cytokine synergy in antigen-independent activation and priming of naive CD8+ T lymphocytes.Crit Rev Immunol. 2009;29(3):219-39. doi: 10.1615/critrevimmunol.v29.i3.30. Crit Rev Immunol. 2009. PMID: 19538136 Review.
-
The signaling symphony: T cell receptor tunes cytokine-mediated T cell differentiation.J Leukoc Biol. 2015 Mar;97(3):477-85. doi: 10.1189/jlb.1RI0614-293R. Epub 2014 Dec 18. J Leukoc Biol. 2015. PMID: 25525115 Free PMC article. Review.
Cited by
-
Befriending the Hostile Tumor Microenvironment in CAR T-Cell Therapy.Front Immunol. 2021 Feb 10;11:618387. doi: 10.3389/fimmu.2020.618387. eCollection 2020. Front Immunol. 2021. PMID: 33643299 Free PMC article. Review.
-
Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations.J Clin Invest. 2020 Mar 2;130(3):1491-1505. doi: 10.1172/JCI132185. J Clin Invest. 2020. PMID: 31830003 Free PMC article. Clinical Trial.
-
Manipulating the TCR signaling network for cellular immunotherapy: Challenges & opportunities.Mol Immunol. 2020 Jul;123:64-73. doi: 10.1016/j.molimm.2020.04.007. Epub 2020 May 15. Mol Immunol. 2020. PMID: 32422416 Free PMC article. Review.
-
Generation and optimization of off-the-shelf immunotherapeutics targeting TCR-Vβ2+ T cell malignancy.Nat Commun. 2024 Jan 15;15(1):519. doi: 10.1038/s41467-024-44786-2. Nat Commun. 2024. PMID: 38225288 Free PMC article.
-
Functional regulation of cytotoxic T cells by gut microbial metabolites.Gut Microbes Rep. 2025;2(1):1-16. doi: 10.1080/29933935.2025.2454002. Epub 2025 Jan 26. Gut Microbes Rep. 2025. PMID: 40115123
References
-
- Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. The Journal of experimental medicine. 1989;170:827–845. - PMC - PubMed
-
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature reviews. Immunology. 2003;3:133–146. - PubMed
-
- Zundler S, Neurath MF. Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev. 2015;26:559–568. - PubMed
-
- Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:734–742. - PubMed
-
- el-Shabrawi Y, Livir-Rallatos C, Christen W, Baltatzis S, Foster CS. High levels of interleukin-12 in the aqueous humor and vitreous of patients with uveitis. Ophthalmology. 1998;105:1659–1663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

