Retinoic acid signaling regulates Krt5 and Krt14 independently of stem cell markers in submandibular salivary gland epithelium
- PMID: 27884045
- PMCID: PMC5348080
- DOI: 10.1002/dvdy.24476
Retinoic acid signaling regulates Krt5 and Krt14 independently of stem cell markers in submandibular salivary gland epithelium
Abstract
Background: Retinoic acid (RA), the active metabolite of vitamin A, has been demonstrated to be important for growth and branching morphogenesis of mammalian embryonic salivary gland epithelium. However, it is not known whether RA functions directly within epithelial cells or in associated tissues that influence morphogenesis of salivary epithelium. Moreover, downstream targets of RA regulation have not been identified.
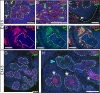
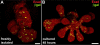
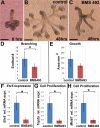
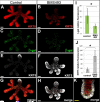
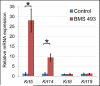
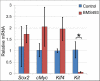
Results: Here, we show that canonical RA signaling occurs in multiple tissues of embryonic mouse salivary glands, including epithelium, associated parasympathetic ganglion neurons, and nonneuronal mesenchyme. By culturing epithelium explants in isolation from other tissues, we demonstrate that RA influences epithelium morphogenesis by direct action in that tissue. Moreover, we demonstrate that inhibition of RA signaling represses cell proliferation and expression of FGF10 signaling targets, and upregulates expression of basal epithelial keratins Krt5 and Krt14. Importantly, we show that the stem cell gene Kit is regulated inversely from Krt5/Krt14 by RA signaling.
Conclusions: RA regulates Krt5 and Krt14 expression independently of stem cell character in developing salivary epithelium. RA, or chemical inhibitors of RA signaling, could potentially be used for modulating growth and differentiation of epithelial stem cells for the purpose of re-populating damaged glands or generating bioengineered organs. Developmental Dynamics 246:135-147, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: KRT5; keratin; progenitor; retinoid; stem cell; vitamin A.
© 2016 Wiley Periodicals, Inc.
Figures
References
-
- Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

