Tsetse fly tolerance to T. brucei infection: transcriptome analysis of trypanosome-associated changes in the tsetse fly salivary gland
- PMID: 27884110
- PMCID: PMC5123318
- DOI: 10.1186/s12864-016-3283-0
Tsetse fly tolerance to T. brucei infection: transcriptome analysis of trypanosome-associated changes in the tsetse fly salivary gland
Abstract
Background: For their transmission, African trypanosomes rely on their blood feeding insect vector, the tsetse fly (Glossina sp.). The ingested Trypanosoma brucei parasites have to overcome a series of barriers in the tsetse fly alimentary tract to finally develop into the infective metacyclic forms in the salivary glands that are transmitted to a mammalian host by the tsetse bite. The parasite population in the salivary gland is dense with a significant number of trypanosomes tightly attached to the epithelial cells. Our current knowledge on the impact of the infection on the salivary gland functioning is very limited. Therefore, this study aimed to gain a deeper insight into the global gene expression changes in the salivary glands of Glossina morsitans morsitans in response to an infection with the T. brucei parasite. A detailed whole transcriptome comparison of midgut-infected tsetse with and without a mature salivary gland infection was performed to study the impact of a trypanosome infection on different aspects of the salivary gland functioning and the mechanisms that are induced in this tissue to tolerate the infection i.e. to control the negative impact of the parasite presence. Moreover, a transcriptome comparison with age-matched uninfected flies was done to see whether gene expression in the salivary glands is already affected by a trypanosome infection in the tsetse midgut.
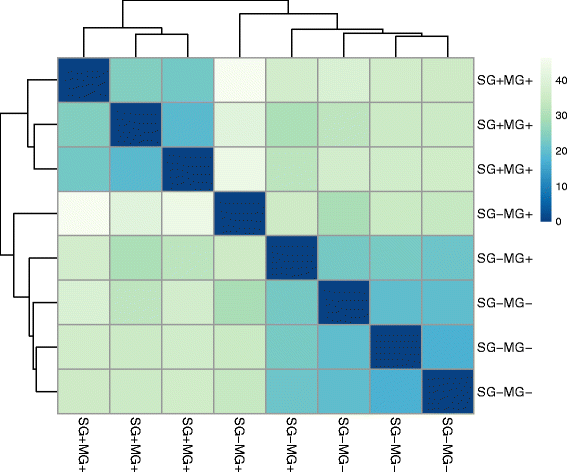
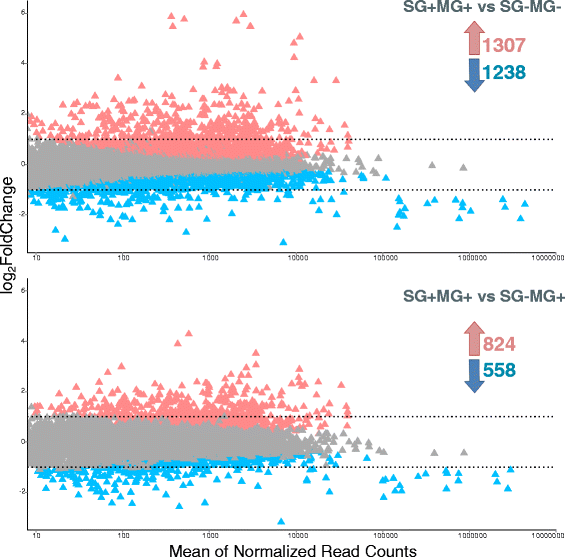
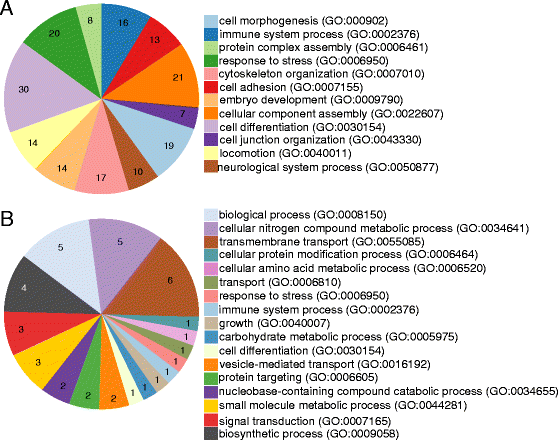
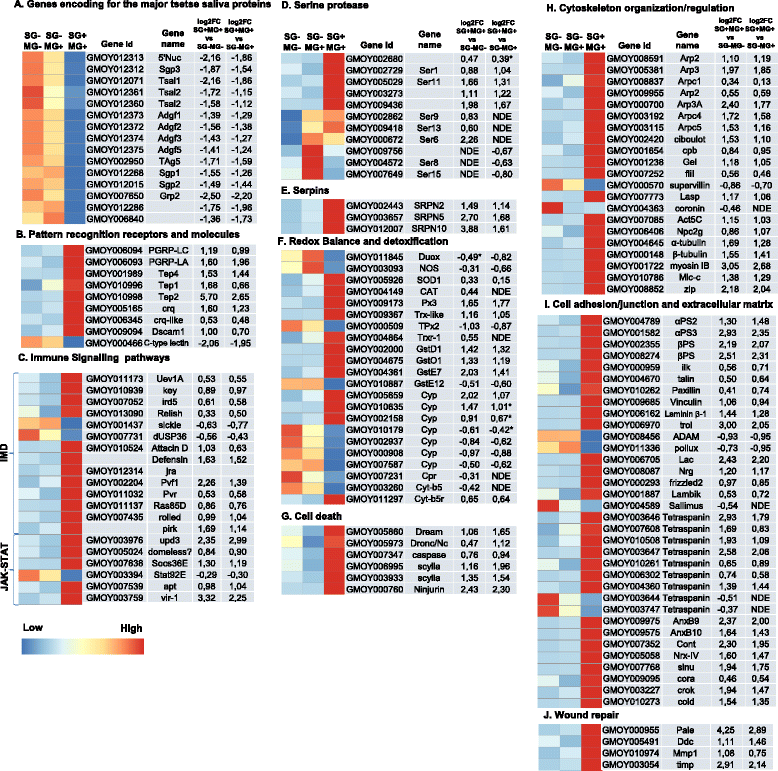
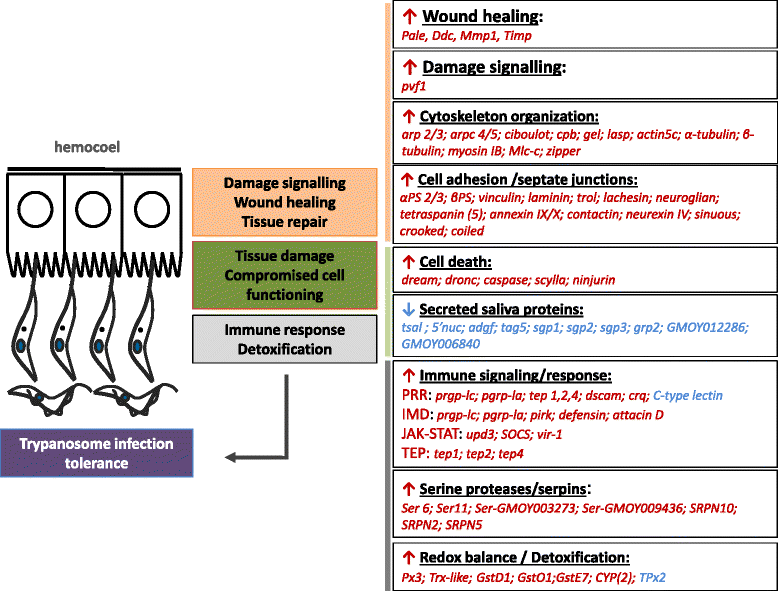
Results: By a RNA-sequencing (RNA-seq) approach we compared the whole transcriptomes of flies with a T. brucei salivary gland/midgut infection versus flies with only a midgut infection or versus non-infected flies, all with the same age and feeding history. More than 7500 salivary gland transcripts were detected from which a core group of 1214 differentially expressed genes (768 up- and 446 down-regulated) were shared between the two transcriptional comparisons. Gene Ontology enrichment analysis and detailed gene expression comparisons showed a diverse impact at the gene transcript level. Increased expression was observed for transcripts encoding for proteins involved in immunity (like several genes of the Imd-signaling pathway, serine proteases, serpins and thioester-containing proteins), detoxification of reactive species, cell death, cytoskeleton organization, cell junction and repair. Decreased expression was observed for transcripts encoding the major secreted proteins such as 5'-nucleotidases, adenosine deaminases and the nucleic acid binding proteins Tsals. Moreover, expression of some gene categories in the salivary glands were found to be already affected by a trypanosome midgut infection, before the parasite reaches the salivary glands.
Conclusions: This study reveals that the T. brucei population in the tsetse salivary gland has a negative impact on its functioning and on the integrity of the gland epithelium. Our RNA-seq data suggest induction of a strong local tissue response in order to control the epithelial cell damage, the ROS intoxication of the cellular environment and the parasite infection, resulting in the fly tolerance to the infection. The modified expression of some gene categories in the tsetse salivary glands by a trypanosome infection at the midgut level indicate a putative anticipatory response in the salivary glands, before the parasite reaches this tissue.
Keywords: RNA-seq; Salivary gland; Tolerance; Trypanosoma brucei; Tsetse fly.
Figures
Similar articles
-
Transcriptome Profiling of Trypanosoma brucei Development in the Tsetse Fly Vector Glossina morsitans.PLoS One. 2016 Dec 21;11(12):e0168877. doi: 10.1371/journal.pone.0168877. eCollection 2016. PLoS One. 2016. PMID: 28002435 Free PMC article.
-
Maturation of a Trypanosoma brucei infection to the infectious metacyclic stage is enhanced in nutritionally stressed tsetse flies.J Med Entomol. 2009 Nov;46(6):1446-9. doi: 10.1603/033.046.0629. J Med Entomol. 2009. PMID: 19960695
-
Insights into the trypanosome-host interactions revealed through transcriptomic analysis of parasitized tsetse fly salivary glands.PLoS Negl Trop Dis. 2014 Apr 24;8(4):e2649. doi: 10.1371/journal.pntd.0002649. eCollection 2014 Apr. PLoS Negl Trop Dis. 2014. PMID: 24763140 Free PMC article.
-
Flying tryps: survival and maturation of trypanosomes in tsetse flies.Trends Parasitol. 2013 Apr;29(4):188-96. doi: 10.1016/j.pt.2013.02.003. Epub 2013 Mar 16. Trends Parasitol. 2013. PMID: 23507033 Review.
-
The Social Life of African Trypanosomes.Trends Parasitol. 2015 Oct;31(10):490-498. doi: 10.1016/j.pt.2015.06.012. Trends Parasitol. 2015. PMID: 26433252 Review.
Cited by
-
Activity of the prophenoloxidase system and survival of triatomines infected with different Trypanosoma cruzi strains under different temperatures: understanding Chagas disease in the face of climate change.Parasit Vectors. 2019 May 8;12(1):219. doi: 10.1186/s13071-019-3477-9. Parasit Vectors. 2019. PMID: 31068226 Free PMC article.
-
Molecular characterization of tsetse's proboscis and its response to Trypanosoma congolense infection.PLoS Negl Trop Dis. 2017 Nov 20;11(11):e0006057. doi: 10.1371/journal.pntd.0006057. eCollection 2017 Nov. PLoS Negl Trop Dis. 2017. PMID: 29155830 Free PMC article.
-
The Tsetse Fly Displays an Attenuated Immune Response to Its Secondary Symbiont, Sodalis glossinidius.Front Microbiol. 2019 Jul 24;10:1650. doi: 10.3389/fmicb.2019.01650. eCollection 2019. Front Microbiol. 2019. PMID: 31396178 Free PMC article.
-
Spiroplasma endosymbiont reduction of host lipid synthesis and Stomoxyn-like peptide contribute to trypanosome resistance in the tsetse fly Glossina fuscipes.bioRxiv [Preprint]. 2024 Oct 24:2024.10.24.620045. doi: 10.1101/2024.10.24.620045. bioRxiv. 2024. Update in: PLoS Pathog. 2025 Jan 31;21(1):e1012692. doi: 10.1371/journal.ppat.1012692. PMID: 39484388 Free PMC article. Updated. Preprint.
-
The Trypanosoma brucei MISP family of invariant proteins is co-expressed with BARP as triple helical bundle structures on the surface of salivary gland forms, but is dispensable for parasite development within the tsetse vector.PLoS Pathog. 2023 Mar 30;19(3):e1011269. doi: 10.1371/journal.ppat.1011269. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36996244 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

