A randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial
- PMID: 27884128
- PMCID: PMC5123375
- DOI: 10.1186/s12884-016-1149-8
A randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial
Erratum in
-
Correction to: A randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial.BMC Pregnancy Childbirth. 2018 May 4;18(1):130. doi: 10.1186/s12884-018-1792-3. BMC Pregnancy Childbirth. 2018. PMID: 29728087 Free PMC article.
Abstract
Background: Maternal obesity is associated with adverse pregnancy outcomes and has lifelong negative implications for offspring health. The Institute of Medicine recommends limited gestational weight gain (GWG) in obese women for optimal maternal and infant outcomes. However, there is a gap regarding an effective and sustainable intervention strategy to achieve this goal. The aim of the healthy mums and babies (HUMBA) demonstration trial is to assess whether a multifaceted nutritional intervention and/or an oral probiotic treatment in obese pregnant women can reduce excessive GWG and optimise pregnancy outcomes.
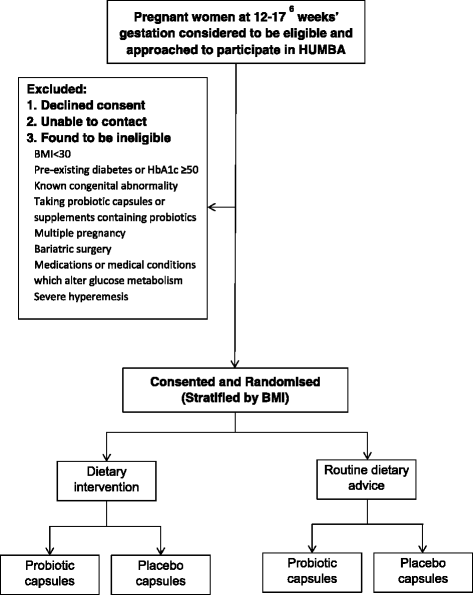
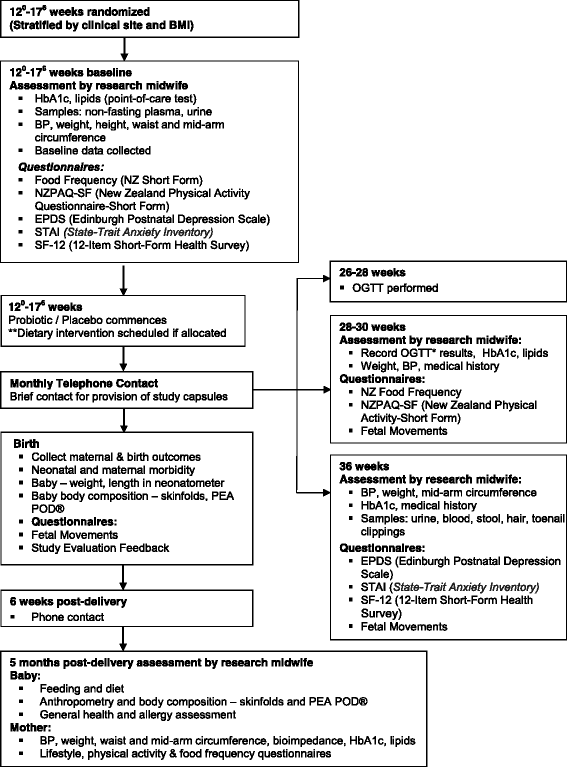
Methods and design: The study is a two by two factorial randomised controlled demonstration trial conducted in Counties Manukau health region, New Zealand, a multi-ethnic region with a high prevalence of obesity. A total of 220 non-diabetic obese women with a singleton pregnancy will be recruited between 120 and 176 weeks. At recruitment, women are randomised to receive either a culturally tailored multifaceted dietary intervention or routine dietary advice, and either an oral probiotic or placebo capsule. Randomisation is undertaken via a web-based protocol, randomize.net, with a 1:1 ratio using stratification by body mass index (BMI) category (BMI of 30-34.9 or BMI ≥35 kg/m2). The dietary intervention includes 4 customised nutrition education visits by a trained community health worker combined with motivational text messaging. Probiotic capsules consist of Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12 at a dose of 7 × 109 colony-forming units one per day until birth. Probiotic and placebo capsules are identically pre-packed and labelled by a third party, and are prescribed in a double blinded fashion. Research assessments are conducted at enrolment, 28 weeks, 36 weeks, at birth and at 5 months post-delivery. The primary outcomes for the study are proportion of women with excessive GWG and infant birthweight.
Discussion: The HUMBA demonstration trial will assess the efficacy of a culturally tailored multifaceted dietary intervention and probiotic treatment in limiting excessive GWG and optimising birthweight in a multiethnic sample of obese pregnant women. If successful, either one or both of the interventions may be incorporated into future studies powered to investigate important pregnancy outcomes.
Trial registration: Australian New Zealand Clinical Trials Registry registration number: ACTRN12615000400561 , Universal Trial Number: U1111-1155-0409. Date registered: 29th April 2015.
Keywords: Birthweight; Gestational weight gain; Nutritional intervention; Obesity; Probiotics; Randomised controlled trial; Study protocol.
Figures
References
-
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. A potential decline in life expectancy in the United States in the 21st century. NEJM. 2005; 352(11):1138–45. - PubMed
-
- OECD. Obesity Update 2014. Paris: OECD; 2014. http://www.oecd.org/els/health-systems/Obesity-Update-2014.pdf. Accessed 28 Dec 2014.
-
- Ministry of Health. Annual Update of Key Results 2014/15: New Zealand Health Survey. Wellington: Ministry of Health; 2015.
-
- Magann EF, Doherty DA, Sandlin AT, Chauhan SP, Morrison JC. The effects of an increasing gradient of maternal obesity on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2013;53(3):250–257. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

