TIGAR cooperated with glycolysis to inhibit the apoptosis of leukemia cells and associated with poor prognosis in patients with cytogenetically normal acute myeloid leukemia
- PMID: 27884166
- PMCID: PMC5123356
- DOI: 10.1186/s13045-016-0360-4
TIGAR cooperated with glycolysis to inhibit the apoptosis of leukemia cells and associated with poor prognosis in patients with cytogenetically normal acute myeloid leukemia
Abstract
Background: Cancer cells show increased glycolysis and take advantage of this metabolic pathway to generate ATP. The TP53-induced glycolysis and apoptosis regulator (TIGAR) inhibits aerobic glycolysis and protects tumor cells from intracellular reactive oxygen species (ROS)-associated apoptosis. However, the function of TIGAR in glycolysis and survival of acute myeloid leukemia cells remains unclear.
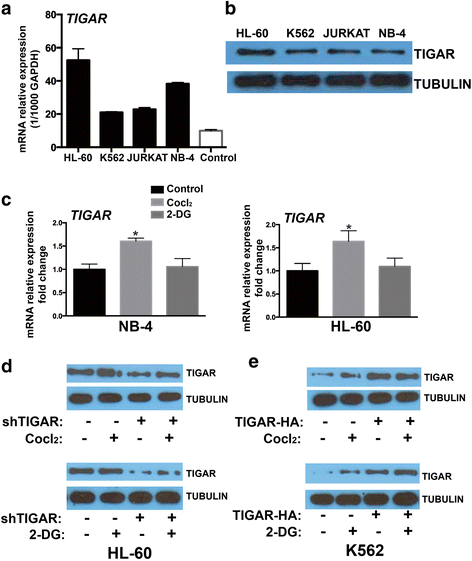
Methods: We analyzed TIGAR expression in cytogenetically normal (CN-) AML patients and the correlations with clinical and biological parameters. In vivo and in vitro, we tested whether glycolysis may induce TIGAR expression and evaluated the combination effect of glycolysis inhibitor and TIGAR knockdown on human leukemia cell proliferation.
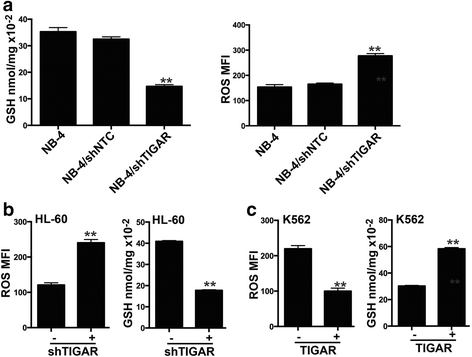
Results: High TIGAR expression was an independent predictor of poor survival and high incidence of relapse in adult patients with CN-AML. TIGAR also showed high expression in multiple human leukemia cell lines and knockdown of TIGAR activated glycolysis through PFKFB3 upregulation in human leukemia cells. Knockdown of TIGAR inhibited the proliferation of human leukemia cells and sensitized leukemia cells to glycolysis inhibitor both in vitro and in vivo. Furthermore, TIGAR knockdown in combination with glycolysis inhibitor 2-DG led leukemia cells to apoptosis. In addition, the p53 activator Nutlin-3α showed a significant combinational effect with TIGAR knockdown in leukemia cells. However, TIGAR expression and its anti-apoptotic effects were uncoupled from overexpression of exogenous p53 in leukemia cells.
Conclusions: TIGAR might be a predictor of poor survival and high incidence of relapse in AML patients, and the combination of TIGAR inhibitors with anti-glycolytic agents may be novel therapies for the future clinical use in AML patients.
Keywords: Acute myeloid leukemia; Apoptosis; Glycolysis; Survival; TIGAR.
Figures





References
-
- Zhao K, Zhou Y, Qiao C, Ni T, Li Z, Wang X, et al. Oroxylin A promotes PTEN-mediated negative regulation of MDM2 transcription via SIRT3-mediated deacetylation to stabilize p53 and inhibit glycolysis in wt-p53 cancer cells. J Hematol Oncol. 2015;8:41. doi: 10.1186/s13045-015-0137-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

