Roux-en-Y gastric bypass augments the feeding responses evoked by gastrin-releasing peptides
- PMID: 27884350
- PMCID: PMC5125518
- DOI: 10.1016/j.jss.2016.08.057
Roux-en-Y gastric bypass augments the feeding responses evoked by gastrin-releasing peptides
Abstract
Background: Roux-en-Y gastric bypass (RYGB) is the most effective method for the treatment of obesity, and metabolic disease RYGB may reduce body weight by altering the feeding responses evoked by the short-term satiety peptides.
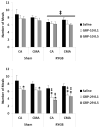
Materials and methods: Here, we measured meal size (MS, chow), intermeal interval (IMI) length, and satiety ratio (SR, IMI/MS; food consumed per a unit of time) by the small and the large forms of gastrin-releasing peptide (GRP) in rats, GRP-10 and GRP-29 (0, 0.1, 0.5 nmol/kg) infused in the celiac artery (CA, supplies stomach and upper duodenum) and the cranial mesenteric artery (CMA, supplies small and large intestine) in an RYGB rat model.
Results: GRP-10 reduced MS, prolonged the IMI, and increased the SR only in the RYGB group, whereas GRP-29 evoked these responses by both routes and in both groups.
Conclusions: The RYGB procedure augments the feeding responses evoked by exogenous GRP, possibly by decreasing total food intake, increasing latency to the first meal, decreasing number of meals or altering the sites of action regulating MS and IMI length by the two peptides.
Keywords: Celiac artery; Cranial mesenteric artery; Food intake; GRP; Roux-en-Y.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
The BB2 receptor antagonist BW2258U89 attenuates the feeding responses evoked by exogenous gastrin releasing peptide-29.Horm Behav. 2016 Sep;85:1-4. doi: 10.1016/j.yhbeh.2016.06.012. Epub 2016 Jul 2. Horm Behav. 2016. PMID: 27381650 Free PMC article.
-
The stomach and/or upper duodenum contain sites of action that control meal size and intermeal interval length by exogenous rat gastrin releasing peptide.Peptides. 2014 May;55:41-6. doi: 10.1016/j.peptides.2014.02.004. Epub 2014 Feb 18. Peptides. 2014. PMID: 24556509
-
Gastrin releasing peptide-29 evokes feeding responses in the rat.Peptides. 2011 Feb;32(2):241-5. doi: 10.1016/j.peptides.2010.10.027. Epub 2010 Nov 3. Peptides. 2011. PMID: 21055429
-
Sequence analysis and feeding responses evoked by the large molecular form of gastrin releasing peptide (GRP) in the rat GRP-29.Peptides. 2014 Sep;59:1-8. doi: 10.1016/j.peptides.2014.06.013. Epub 2014 Jun 30. Peptides. 2014. PMID: 24993846
-
The role of bombesin and bombesin-related peptides in the short-term control of food intake.Prog Mol Biol Transl Sci. 2013;114:343-70. doi: 10.1016/B978-0-12-386933-3.00010-8. Prog Mol Biol Transl Sci. 2013. PMID: 23317790 Review.
Cited by
-
The Phantom Satiation Hypothesis of Bariatric Surgery.Front Neurosci. 2021 Feb 1;15:626085. doi: 10.3389/fnins.2021.626085. eCollection 2021. Front Neurosci. 2021. PMID: 33597843 Free PMC article.
-
Catheter-guided anvil insertion for circular stapler esophagojejunal anastomosis: a novel technique in laparoscopic total gastrectomy.Updates Surg. 2024 Aug;76(4):1547-1552. doi: 10.1007/s13304-024-01753-2. Epub 2024 Mar 7. Updates Surg. 2024. PMID: 38451410 Free PMC article.
-
Peptide Tyrosine Tyrosine 3-36 Reduces Meal Size and Activates the Enteric Neurons in Male Sprague-Dawley Rats.Dig Dis Sci. 2017 Dec;62(12):3350-3358. doi: 10.1007/s10620-017-4788-3. Epub 2017 Oct 13. Dig Dis Sci. 2017. PMID: 29030744
-
Recent Advances in the Neurobiology of Altered Motivation Following Bariatric Surgery.Curr Psychiatry Rep. 2019 Nov 9;21(11):117. doi: 10.1007/s11920-019-1084-2. Curr Psychiatry Rep. 2019. PMID: 31707546 Review.
References
-
- Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. American journal of surgery. 2002;184:9S–16S. - PubMed
-
- Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. International journal of obesity. 2009;33(Suppl 1):S33–40. - PubMed
-
- Wang Y, Liu J. Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. Obesity surgery. 2009;19:357–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

