Impact of external pneumatic compression target inflation pressure on transcriptome-wide RNA expression in skeletal muscle
- PMID: 27884954
- PMCID: PMC5357997
- DOI: 10.14814/phy2.13029
Impact of external pneumatic compression target inflation pressure on transcriptome-wide RNA expression in skeletal muscle
Abstract
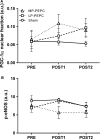
Next-generation RNA sequencing was employed to determine the acute and subchronic impact of peristaltic pulse external pneumatic compression (PEPC) of different target inflation pressures on global gene expression in human vastus lateralis skeletal muscle biopsy samples. Eighteen (N = 18) male participants were randomly assigned to one of the three groups: (1) sham (n = 6), 2) EPC at 30-40 mmHg (LP-EPC; n = 6), and 3) EPC at 70-80 mmHg (MP-EPC; n = 6). One hour treatment with sham/EPC occurred for seven consecutive days. Vastus lateralis skeletal muscle biopsies were performed at baseline (before first treatment; PRE), 1 h following the first treatment (POST1), and 24 h following the last (7th) treatment (POST2). Changes from PRE in gene expression were analyzed via paired comparisons within each group. Genes were filtered to include only those that had an RPKM ≥ 1.0, a fold-change of ≥1.5 and a paired t-test value of <0.01. For the sham condition, two genes at POST1 and one gene at POST2 were significantly altered. For the LP-EPC condition, nine genes were up-regulated and 0 genes were down-regulated at POST1 while 39 genes were up-regulated and one gene down-regulated at POST2. For the MP-EPC condition, two genes were significantly up-regulated and 21 genes were down-regulated at POST1 and 0 genes were altered at POST2. Both LP-EPC and MP-EPC acutely alter skeletal muscle gene expression, though only LP-EPC appeared to affect gene expression with subchronic application. Moreover, the transcriptome response to EPC demonstrated marked heterogeneity (i.e., genes and directionality) with different target inflation pressures.
Keywords: External pneumatic compression; PGC‐1α; RNA sequencing; redox balance; skeletal muscle.
© 2016 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures




References
-
- Cohen, J. 1992. A power primer. Psychol. Bull. 112:155–159. - PubMed
-
- Fang, Z. , and Cui X.. 2011. Design and validation issues in RNA‐seq experiments. Brief. Bioinform 12:280–287. - PubMed
-
- Ferrara, N. , and Davis‐Smyth T.. 1997. The biology of vascular endothelial growth factor. Endocr. Rev. 18:4–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

