The emerging role of immune checkpoint inhibition in malignant lymphoma
- PMID: 27884973
- PMCID: PMC5210230
- DOI: 10.3324/haematol.2016.150656
The emerging role of immune checkpoint inhibition in malignant lymphoma
Abstract
To evade elimination by the host immune system, tumor cells commonly exploit physiological immune checkpoint pathways, restraining efficient anti-tumor immune cell function. Growing understanding of the complex dialog between tumor cells and their microenvironment contributed to the development of immune checkpoint inhibitors. This innovative strategy has demonstrated paradigm-shifting clinical activity in various malignancies. Antibodies targeting programmed death 1 and cytotoxic T-lymphocyte-associated protein-4 are also being investigated in lymphoid malignancies with varying levels of activity and a favorable toxicity profile. To date, evaluated only in the setting of relapsed or refractory disease, anti-programmed death 1 antibodies such as nivolumab and pembrolizumab show encouraging response rates particularly in classical Hodgkin lymphoma but also in follicular lymphoma and diffuse-large B-cell lymphoma. As the first immune checkpoint inhibitor in lymphoma, nivolumab was approved for the treatment of relapsed or refractory classical Hodgkin lymphoma by the Food and Drug Administration in May 2016. In this review, we assess the role of the pathways involved and potential rationale of checkpoint inhibition in various lymphoid malignancies. In addition to data from current clinical trials, immune-related side effects, potential limitations and future perspectives including promising combinatory approaches with immune checkpoint inhibition are discussed.
Copyright© Ferrata Storti Foundation.
Figures


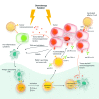
References
-
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. IARC CancerBase No 11 2013. [cited 2016 29.04.2016.]; Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx
-
- Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition. Lyon, France: IARC Press, 2008.
-
- Hainsworth JD. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin’s lymphoma: interim follow-up of a multicenter phase II trial. Semin Oncol. 2002;29(1 Suppl 2):25–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

