Directed evolution of cytochrome c for carbon-silicon bond formation: Bringing silicon to life
- PMID: 27885032
- PMCID: PMC5243118
- DOI: 10.1126/science.aah6219
Directed evolution of cytochrome c for carbon-silicon bond formation: Bringing silicon to life
Abstract
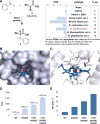
Enzymes that catalyze carbon-silicon bond formation are unknown in nature, despite the natural abundance of both elements. Such enzymes would expand the catalytic repertoire of biology, enabling living systems to access chemical space previously only open to synthetic chemistry. We have discovered that heme proteins catalyze the formation of organosilicon compounds under physiological conditions via carbene insertion into silicon-hydrogen bonds. The reaction proceeds both in vitro and in vivo, accommodating a broad range of substrates with high chemo- and enantioselectivity. Using directed evolution, we enhanced the catalytic function of cytochrome c from Rhodothermus marinus to achieve more than 15-fold higher turnover than state-of-the-art synthetic catalysts. This carbon-silicon bond-forming biocatalyst offers an environmentally friendly and highly efficient route to producing enantiopure organosilicon molecules.
Copyright © 2016, American Association for the Advancement of Science.
Figures


Comment in
-
Teaching nature the unnatural.Science. 2016 Nov 25;354(6315):970. doi: 10.1126/science.aal1951. Science. 2016. PMID: 27884992 No abstract available.
Similar articles
-
Origin and Control of Chemoselectivity in Cytochrome c Catalyzed Carbene Transfer into Si-H and N-H bonds.J Am Chem Soc. 2021 May 12;143(18):7114-7123. doi: 10.1021/jacs.1c02146. Epub 2021 Apr 28. J Am Chem Soc. 2021. PMID: 33909977 Free PMC article.
-
Genetically programmed chiral organoborane synthesis.Nature. 2017 Dec 7;552(7683):132-136. doi: 10.1038/nature24996. Epub 2017 Nov 29. Nature. 2017. PMID: 29186119 Free PMC article.
-
Improving the Long-term Enantioselectivity of a Silicon-Carbon Bond-Forming Enzyme.Chemistry. 2025 Apr 1;31(19):e202404688. doi: 10.1002/chem.202404688. Epub 2025 Mar 16. Chemistry. 2025. PMID: 39876675
-
Directed Evolution: Bringing New Chemistry to Life.Angew Chem Int Ed Engl. 2018 Apr 9;57(16):4143-4148. doi: 10.1002/anie.201708408. Epub 2017 Nov 28. Angew Chem Int Ed Engl. 2018. PMID: 29064156 Free PMC article. Review.
-
Discovery and synthetic applications of novel silicon-carbon bond cleavage reactions based on the coordination number change of organosilicon compounds.Proc Jpn Acad Ser B Phys Biol Sci. 2008;84(5):123-33. doi: 10.2183/pjab.84.123. Proc Jpn Acad Ser B Phys Biol Sci. 2008. PMID: 18941292 Free PMC article. Review.
Cited by
-
Origin and Control of Chemoselectivity in Cytochrome c Catalyzed Carbene Transfer into Si-H and N-H bonds.J Am Chem Soc. 2021 May 12;143(18):7114-7123. doi: 10.1021/jacs.1c02146. Epub 2021 Apr 28. J Am Chem Soc. 2021. PMID: 33909977 Free PMC article.
-
Simultaneous Improvement of Final Product-Tolerance and Thermostability of GH39 Xylosidase for Prebiotic Production by Directed Evolution.Foods. 2022 Sep 30;11(19):3039. doi: 10.3390/foods11193039. Foods. 2022. PMID: 36230114 Free PMC article.
-
Recent Advances in Asymmetric Iron Catalysis.Molecules. 2020 Aug 26;25(17):3889. doi: 10.3390/molecules25173889. Molecules. 2020. PMID: 32858925 Free PMC article. Review.
-
Enhanced Sequence-Activity Mapping and Evolution of Artificial Metalloenzymes by Active Learning.ACS Cent Sci. 2024 May 22;10(7):1357-1370. doi: 10.1021/acscentsci.4c00258. eCollection 2024 Jul 24. ACS Cent Sci. 2024. PMID: 39071060 Free PMC article.
-
A multi-functional genetic manipulation system and its use in high-level expression of a β-mannanase mutant with high specific activity in Pichia pastoris.Microb Biotechnol. 2021 Jul;14(4):1525-1538. doi: 10.1111/1751-7915.13812. Epub 2021 May 4. Microb Biotechnol. 2021. PMID: 33942496 Free PMC article.
References
-
- Frampton MB, Zelisko PM. Organosilicon biotechnology. Silicon. 2009;1:147–163.
-
- Rappoport Z, Apeloig Y, editors. The Chemistry of Organic Silicon Compounds. Vol. 3 Wiley; 2003.
-
- Ponomarenko SA, Kirchmeyer S. Conjugated organosilicon materials for organic electronics and photonics. Adv Polym Sci. 2011;235:33–110.
-
- Showell GA, Mills JS. Chemistry challenges in lead optimization: Silicon isosteres in drug discovery. Drug Discov Today. 2003;8:551–556. - PubMed
-
- Franz AK, Wilson SO. Organosilicon molecules with medicinal applications. J Med Chem. 2013;56:388–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

