Neoadjuvant chemotherapy in breast cancers
- PMID: 27885165
- PMCID: PMC5373271
- DOI: 10.1177/1745505716677139
Neoadjuvant chemotherapy in breast cancers
Abstract
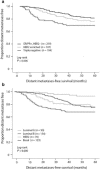
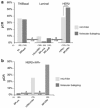
With advances in science and technology, there are more innovations in the approach to management of patients with breast cancer. Neoadjuvant chemotherapy that is designed to be used prior to surgical removal of a tumor has received significant attention. Currently, neoadjuvant chemotherapy is offered to patients with locally advanced breast cancer and also those breast cancer patients who may benefit from size reduction before conservation therapy. There is now sufficient evidence that if neoadjuvant chemotherapy leads to complete pathologic response, the patient will enjoy a better outcome. Therefore, assessment of the degree of response to neoadjuvant chemotherapy has a major impact on patient selection and the follow-up management of each patient and defines patient outcome.
Keywords: breast cancer; breast pathology report; neoadjuvant chemotherapy.
© The Author(s) 2016.
Conflict of interest statement
Figures








References
-
- Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 1999; 17(2): 460–469. - PubMed
-
- Smith IE, Lipton L. Preoperative/neoadjuvant medical therapy for early breast cancer. Lancet Oncol 2001; 2: 561–570. - PubMed
-
- Kaufmann M, Von Minckwitz G, Smith R, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol 2003; 21(13): 2600–2608. - PubMed
-
- Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 2005; 97: 188–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

