Nitric oxide triggers a transient metabolic reprogramming in Arabidopsis
- PMID: 27885260
- PMCID: PMC5122866
- DOI: 10.1038/srep37945
Nitric oxide triggers a transient metabolic reprogramming in Arabidopsis
Abstract
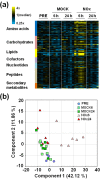
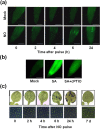
Nitric oxide (NO) regulates plant growth and development as well as responses to stress that enhanced its endogenous production. Arabidopsis plants exposed to a pulse of exogenous NO gas were used for untargeted global metabolomic analyses thus allowing the identification of metabolic processes affected by NO. At early time points after treatment, NO scavenged superoxide anion and induced the nitration and the S-nitrosylation of proteins. These events preceded an extensive though transient metabolic reprogramming at 6 h after NO treatment, which included enhanced levels of polyamines, lipid catabolism and accumulation of phospholipids, chlorophyll breakdown, protein and nucleic acid turnover and increased content of sugars. Accordingly, lipid-related structures such as root cell membranes and leaf cuticle altered their permeability upon NO treatment. Besides, NO-treated plants displayed degradation of starch granules, which is consistent with the increased sugar content observed in the metabolomic survey. The metabolic profile was restored to baseline levels at 24 h post-treatment, thus pointing up the plasticity of plant metabolism in response to nitroxidative stress conditions.
Figures





References
-
- Beligni M. V. & Lamattina L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210, 215–221 (2000). - PubMed
-
- He Y. et al.. Nitric oxide represses the Arabidopsis floral transition. Science 305, 1968–1971 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

