Multiscale Opening of Conjoined Fuzzy Objects: Theory and Applications
- PMID: 27885318
- PMCID: PMC5116813
- DOI: 10.1109/TFUZZ.2015.2502278
Multiscale Opening of Conjoined Fuzzy Objects: Theory and Applications
Abstract
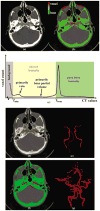
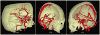
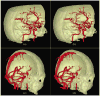
Theoretical properties of a multi-scale opening (MSO) algorithm for two conjoined fuzzy objects are established, and its extension to separating two conjoined fuzzy objects with different intensity properties is introduced. Also, its applications to artery/vein (A/V) separation in pulmonary CT imaging and carotid vessel segmentation in CT angiograms (CTAs) of patients with intracranial aneurysms are presented. The new algorithm accounts for distinct intensity properties of individual conjoined objects by combining fuzzy distance transform (FDT), a morphologic feature, with fuzzy connectivity, a topologic feature. The algorithm iteratively opens the two conjoined objects starting at large scales and progressing toward finer scales. Results of application of the method in separating arteries and veins in a physical cast phantom of a pig lung are presented. Accuracy of the algorithm is quantitatively evaluated in terms of sensitivity and specificity on patients' CTA data sets and its performance is compared with existing methods. Reproducibility of the algorithm is examined in terms of volumetric agreement between two users' carotid vessel segmentation results. Experimental results using this algorithm on patients' CTA data demonstrate a high average accuracy of 96.3% with 95.1% sensitivity and 97.5% specificity and a high reproducibility of 94.2% average agreement between segmentation results from two mutually independent users. Approximately, twenty-five to thirty-five user-specified seeds/separators are needed for each CTA data through a custom designed graphical interface requiring an average of thirty minutes to complete carotid vascular segmentation in a patient's CTA data set.
Keywords: CTA; Fuzzy connectivity; carotid vasculature; fuzzy distance transform; morphology; multi-scale opening; pulmonary vasculature.
Figures





Similar articles
-
Topomorphologic separation of fused isointensity objects via multiscale opening: separating arteries and veins in 3-D pulmonary CT.IEEE Trans Med Imaging. 2010 Mar;29(3):840-51. doi: 10.1109/TMI.2009.2038224. IEEE Trans Med Imaging. 2010. PMID: 20199919 Free PMC article.
-
A new paradigm of interactive artery/vein separation in noncontrast pulmonary CT imaging using multiscale topomorphologic opening.IEEE Trans Biomed Eng. 2012 Nov;59(11):3016-27. doi: 10.1109/TBME.2012.2212894. Epub 2012 Aug 10. IEEE Trans Biomed Eng. 2012. PMID: 22899571 Free PMC article.
-
Quantification of coronary arterial stenoses in CTA using fuzzy distance transform.Comput Med Imaging Graph. 2012 Jan;36(1):11-24. doi: 10.1016/j.compmedimag.2011.03.004. Epub 2011 May 8. Comput Med Imaging Graph. 2012. PMID: 21555207
-
A New Algorithm for Cortical Bone Segmentation with Its Validation and Applications to In Vivo Imaging.Proc Int Conf Image Anal Process. 2013 Sep;8157:349-358. doi: 10.1007/978-3-642-41184-7_36. Proc Int Conf Image Anal Process. 2013. PMID: 27398415 Free PMC article.
-
Automatic segmentation of intracranial arteries and veins in four-dimensional cerebral CT perfusion scans.Med Phys. 2010 Jun;37(6):2956-66. doi: 10.1118/1.3397813. Med Phys. 2010. PMID: 20632608
Cited by
-
Quantitative 3-D morphometric analysis of individual dendritic spines.Sci Rep. 2018 Feb 23;8(1):3545. doi: 10.1038/s41598-018-21753-8. Sci Rep. 2018. PMID: 29476060 Free PMC article.
-
PartSeg: a tool for quantitative feature extraction from 3D microscopy images for dummies.BMC Bioinformatics. 2021 Feb 17;22(1):72. doi: 10.1186/s12859-021-03984-1. BMC Bioinformatics. 2021. PMID: 33596823 Free PMC article.
References
-
- Bushberg JT, Seibert JA, Leidholdt EM, Jr, Boone JM. The Essential Physics of Medical Imaging. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
-
- Cho ZH, Jones JP, Singh M. Foundation of Medical Imaging. New York: Wiley; 1993.
-
- Saha PK, Chaudhuri BB, Majumder DD. A new shape preserving parallel thinning algorithm for 3D digital images. Pat Recog. 1997;30:1939–1955.
-
- Saha PK, Strand R, Borgefors G. Digital topology and geometry in medical imaging: A survey. IEEE Trans Med Imag. 2015;34(9):1940–1964. - PubMed
-
- Saha PK, Borgefors G, Sanniti di Baja G. A survey on skeletonization algorithms and their applications. Pat Recog Lett. in press.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
