Contested Issues of Efficacy and Safety between Transnational Formulation Regimes of Tibetan Medicines in China and Europe
- PMID: 27885323
- PMCID: PMC5119578
- DOI: 10.1163/15734218-12341360
Contested Issues of Efficacy and Safety between Transnational Formulation Regimes of Tibetan Medicines in China and Europe
Abstract
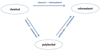
Tibetan medicines are key material objects for medical treatment and have become part of a global trend of 'pharmaceuticalisation', playing increasingly important political and socio-economic roles in an 'alternative modernity'. As I argue in this paper, they also have become key 'sites of contestation' between different epistemic values and styles of practice related to efficacy and safety that are reproduced in and through specific formulation regimes. Based on my multisited ethnography of production, prescription, and use practices of Tibetan medicines in China and Europe, this paper conceptualises three distinct formulation regimes, offering a heuristic model for transnational comparison-a classical, an industrialised or reformulated, and a polyherbal regime. The first two are the major orientations while the polyherbal is a conjoint hybrid with either the classical or the industrialised formulation regime. Globalised national drug safety regulations legalise and confer legitimacy to industrialised Tibetan formulas that follow biomedically defined efficacy, safety, and disease categories, while classical formulas produced by private physicians or small-scale cottage pharmacies are increasingly marginalised as producing 'unsafe' and at times illegal medicines, and need to find new ways for adapting and circulating their formulas.
Keywords: China; Europe; Tibetan medicines; efficacy; legality; safety; transnational formulation regimes.
Figures




References
-
- Adams V. The Sacred in the Scientific: Ambiguous Practices of Science in Tibetan Medicine. Cultural Anthropology. 2002a;16(4):542–75. - PubMed
-
- Adams V. Establishing Proof: Translating “Science” and the State in Tibetan Medicine. In: Lock M, Nichter M, editors. New Horizons in Medical Anthropology: Essays in Honour of Charles Leslie. London: Bergin and Garvey; 2002b. pp. 200–20.
-
- Adams V, Miller S, Craig S, Samen A, Nyima, Sonam, Droyoung, Lhakpen, Varner M. The Challenge of Cross-Cultural Clinical Trials Research: Case Report from the Tibetan Autonomous Region, People’s Republic of China. Medical Anthropology Quarterly. 2005;19(3):267–89. - PubMed
-
- Adams V, Le P, Dhondup R. Translating Science: The Arura Medical Group at the Frontiers of Medical Research. In: Craig S, Cuomu M, Garrett F, Schrempf M, editors. Studies of Medical Pluralism in Tibetan History and Society. Andiast: International Institute for Tibetan and Buddhist Studies GmbH; 2010. pp. 111–36. Proceedings of the 11th Seminar of the International Association for Tibetan Studies, Bonn 2006.
-
- Adams V, Le P, Dhondup R. A Tibetan Way of Science: Revisioning Biomedicine as Tibetan Practice. In: Adams V, Schrempf M, Craig S, editors. Medicine between Science and Religion. 2011. pp. 107–26.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
