PrEP Awareness, Familiarity, Comfort, and Prescribing Experience among US Primary Care Providers and HIV Specialists
- PMID: 27885552
- PMCID: PMC5500978
- DOI: 10.1007/s10461-016-1625-1
PrEP Awareness, Familiarity, Comfort, and Prescribing Experience among US Primary Care Providers and HIV Specialists
Abstract
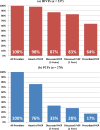
HIV pre-exposure prophylaxis (PrEP) was FDA approved in 2012, but uptake remains low. To characterize what would facilitate health care providers' increased PrEP prescribing, we conducted a 10-city, online survey of 525 primary care providers (PCPs) and HIV providers (HIVPs) to assess awareness, knowledge, and experience with prescribing PrEP; and, comfort with and barriers to PrEP-related activities. Fewer PCPs than HIVPs had heard of PrEP (76 vs 98%), felt familiar with prescribing PrEP (28 vs. 76%), or had prescribed it (17 vs. 64%). PCPs were less comfortable than HIVPs with PrEP-related activities such as discussing sexual activities (75 vs. 94%), testing for acute HIV (83 vs. 98%), or delivering a new HIV diagnosis (80 vs. 95%). PCPs most frequently identified limited knowledge about PrEP and concerns about insurance coverage as prescribing barriers. PCPs and HIVPs differ in needs that will facilitate their PrEP prescribing. Efforts to increase PrEP uptake will require interventions to increase the knowledge, comfort, and skills of providers to prescribe PrEP.
Keywords: Barriers; HIV pre-exposure prophylaxis; HIV prevention; Health care providers.
Conflict of interest statement
A.P. receives research support from Gilead Sciences, Inc. J.W., J.O., T.M., L.B., and J.K. report no conflict of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This study was reviewed by the Institutional Review Board of the Medical College of Wisconsin. It met the Board’s definition of “minimal risk” and a waiver of informed consent was granted.
Figures
Similar articles
-
An educational initiative in response to identified PrEP prescribing needs among PCPs in the Southern U.S.AIDS Care. 2018 May;30(5):650-655. doi: 10.1080/09540121.2017.1384534. Epub 2017 Oct 3. AIDS Care. 2018. PMID: 28971705 Free PMC article.
-
A Cross-Sectional Online Survey of HIV Pre-Exposure Prophylaxis Adoption Among Primary Care Physicians.J Gen Intern Med. 2017 Jan;32(1):62-70. doi: 10.1007/s11606-016-3903-z. Epub 2016 Oct 24. J Gen Intern Med. 2017. PMID: 27778215 Free PMC article.
-
Knowledge, Practices, and Barriers to HIV Preexposure Prophylaxis Prescribing Among Washington State Medical Providers.Sex Transm Dis. 2018 Jul;45(7):452-458. doi: 10.1097/OLQ.0000000000000781. Sex Transm Dis. 2018. PMID: 29465664
-
Health Care Provider Barriers to HIV Pre-Exposure Prophylaxis in the United States: A Systematic Review.AIDS Patient Care STDS. 2020 Mar;34(3):111-123. doi: 10.1089/apc.2019.0189. Epub 2020 Feb 28. AIDS Patient Care STDS. 2020. PMID: 32109141 Free PMC article.
-
HIV Pre-Exposure Prophylaxis Implementation Cascade Among Health Care Professionals in the United States: Implications from a Systematic Review and Meta-Analysis.AIDS Patient Care STDS. 2019 Dec;33(12):507-527. doi: 10.1089/apc.2019.0119. AIDS Patient Care STDS. 2019. PMID: 31821044 Free PMC article.
Cited by
-
A more practical guide to incorporating health equity domains in implementation determinant frameworks.Implement Sci Commun. 2021 Jun 5;2(1):61. doi: 10.1186/s43058-021-00146-5. Implement Sci Commun. 2021. PMID: 34090524 Free PMC article.
-
Concomitant Utilization of Pre-Exposure Prophylaxis (PrEP) and Meningococcal Vaccine (MenACWY) Among Gay, Bisexual, and Other Men Who Have Sex with Men in Los Angeles County, California.Arch Sex Behav. 2020 Jan;49(1):137-146. doi: 10.1007/s10508-019-01500-4. Epub 2019 Oct 18. Arch Sex Behav. 2020. PMID: 31628630 Free PMC article.
-
Health Care Provider Decisions to Initiate Oral HIV Preexposure Prophylaxis in New York City Public Sexual Health Clinics.Sex Transm Dis. 2023 Jun 1;50(6):386-394. doi: 10.1097/OLQ.0000000000001782. Epub 2023 Feb 8. Sex Transm Dis. 2023. PMID: 36749905 Free PMC article.
-
Changes in Cost and Insurance Challenges to Cover PrEP Between 2019 and 2021.J Acquir Immune Defic Syndr. 2023 Jun 1;93(2):116-125. doi: 10.1097/QAI.0000000000003180. J Acquir Immune Defic Syndr. 2023. PMID: 36881835 Free PMC article.
-
Training health care providers to provide PrEP for HIV serodiscordant couples attending public health facilities in Kenya.Glob Public Health. 2019 Oct;14(10):1524-1534. doi: 10.1080/17441692.2019.1588908. Epub 2019 Mar 14. Glob Public Health. 2019. PMID: 30871413 Free PMC article.
References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. - PubMed
-
- Molina J, Capitant C, Spire B, Pialoux G, Chidiac C, Charreau I, et al. On demand PrEP with oral TDF-FTC in MSM: results of the ANRS Ipergay trial. Conference on Retroviruses and Opportunistic Infections; Seattle. 2015; [Abstract 23LB]
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous