Gram-Scale Synthesis of Chiral Cyclopropane-Containing Drugs and Drug Precursors with Engineered Myoglobin Catalysts Featuring Complementary Stereoselectivity
- PMID: 27885768
- PMCID: PMC5755974
- DOI: 10.1002/anie.201608680
Gram-Scale Synthesis of Chiral Cyclopropane-Containing Drugs and Drug Precursors with Engineered Myoglobin Catalysts Featuring Complementary Stereoselectivity
Abstract
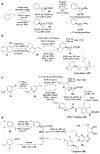
Engineered hemoproteins have recently emerged as promising systems for promoting asymmetric cyclopropanations, but variants featuring predictable, complementary stereoselectivity in these reactions have remained elusive. In this study, a rationally driven strategy was implemented and applied to engineer myoglobin variants capable of providing access to 1-carboxy-2-aryl-cyclopropanes with high trans-(1R,2R) selectivity and catalytic activity. The stereoselectivity of these cyclopropanation biocatalysts complements that of trans-(1S,2S)-selective variants developed here and previously. In combination with whole-cell biotransformations, these stereocomplementary biocatalysts enabled the multigram synthesis of the chiral cyclopropane core of four drugs (Tranylcypromine, Tasimelteon, Ticagrelor, and a TRPV1 inhibitor) in high yield and with excellent diastereo- and enantioselectivity (98-99.9% de; 96-99.9% ee). These biocatalytic strategies outperform currently available methods to produce these drugs.
Keywords: asymmetric cyclopropanation; biocatalysis; carbenoids; myoglobin; protein engineering.
© 2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Talele TT. J Med Chem. 2016;59(19):8712–8756. - PubMed
-
-
Doyle MP, Forbes DC. Chem Rev. 1998;98:911–936.Lebel H, Marcoux JF, Molinaro C, Charette AB. Chem Rev. 2003;103:977–1050.Pellissier H. Tetrahedron. 2008;64:7041–7095.Lowenthal RE, Abiko A, Masamune S. Tetrahedron Lett. 1990;31:6005–6008.For representative examples with acceptor-only diazo reagents: Evans DA, Woerpel KA, Hinman MM, Faul MM. J Am Chem Soc. 1991;113:726–728.Doyle MP, Winchester WR, Hoorn JAA, Lynch V, Simonsen SH, Ghosh R. J Am Chem Soc. 1993;115:9968–9978.Nishiyama H, Itoh Y, Matsumoto H, Park SB, Itoh K. J Am Chem Soc. 1994;116:2223–2224.Uchida T, Irie R, Katsuki T. Synlett. 1999:1163–1165.Che CM, Huang JS, Lee FW, Li Y, Lai TS, Kwong HL, Teng PF, Lee WS, Lo WC, Peng SM, Zhou ZY. J Am Chem Soc. 2001;123:4119–4129.Huang LY, Chen Y, Gao GY, Zhang XP. J Org Chem. 2003;68:8179–8184.Fantauzzi S, Gallo E, Rose E, Raoul N, Caselli A, Issa S, Ragaini F, Cenini S. Organometallics. 2008;27:6143–6151.Carminati DM, Intrieri D, Caselli A, Le Gac S, Boitrel B, Toma L, Legnani L, Gallo E. Chemistry. 2016;22:13599–13612.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

