Optical Aptamer Probes of Fluorescent Imaging to Rapid Monitoring of Circulating Tumor Cell
- PMID: 27886058
- PMCID: PMC5134568
- DOI: 10.3390/s16111909
Optical Aptamer Probes of Fluorescent Imaging to Rapid Monitoring of Circulating Tumor Cell
Abstract
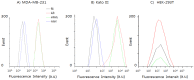
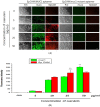
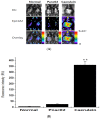
Fluorescence detecting of exogenous EpCAM (epithelial cell adhesion molecule) or muc1 (mucin1) expression correlated to cancer metastasis using nanoparticles provides pivotal information on CTC (circulating tumor cell) occurrence in a noninvasive tool. In this study, we study a new skill to detect extracellular EpCAM/muc1 using quantum dot-based aptamer beacon (QD-EpCAM/muc1 ALB (aptamer linker beacon). The QD-EpCAM/muc1 ALB was designed using QDs (quantum dots) and probe. The EpCAM/muc1-targeting aptamer contains a Ep-CAM/muc1 binding sequence and BHQ1 (black hole quencher 1) or BHQ2 (black hole quencher2). In the absence of target EpCAM/muc1, the QD-EpCAM/muc1 ALB forms a partial duplex loop-like aptamer beacon and remained in quenched state because the BHQ1/2 quenches the fluorescence signal-on of the QD-EpCAM/muc1 ALB. The binding of EpCAM/muc1 of CTC to the EpCAM/muc1 binding aptamer sequence of the EpCAM/muc1-targeting oligonucleotide triggered the dissociation of the BHQ1/2 quencher and subsequent signal-on of a green/red fluorescence signal. Furthermore, acute inflammation was stimulated by trigger such as caerulein in vivo, which resulted in increased fluorescent signal of the cy5.5-EpCAM/muc1 ALB during cancer metastasis due to exogenous expression of EpCAM/muc1 in Panc02-implanted mouse model.
Keywords: ALB; EpCAM; aptamer; metastasis; molecular beacon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Quantum dot-based molecular beacon to monitor intracellular microRNAs.Sensors (Basel). 2015 Jun 2;15(6):12872-83. doi: 10.3390/s150612872. Sensors (Basel). 2015. PMID: 26043176 Free PMC article.
-
Aptamer-based detection of epithelial tumor marker mucin 1 with quantum dot-based fluorescence readout.Anal Chem. 2009 Aug 1;81(15):6130-9. doi: 10.1021/ac901223q. Anal Chem. 2009. PMID: 19572710
-
A novel aptamer functionalized CuInS2 quantum dots probe for daunorubicin sensing and near infrared imaging of prostate cancer cells.Anal Chim Acta. 2014 Mar 25;818:54-60. doi: 10.1016/j.aca.2014.01.057. Epub 2014 Feb 7. Anal Chim Acta. 2014. PMID: 24626403
-
Anti-MUC1 aptamer: A potential opportunity for cancer treatment.Med Res Rev. 2017 Nov;37(6):1518-1539. doi: 10.1002/med.21462. Epub 2017 Jul 31. Med Res Rev. 2017. PMID: 28759115 Review.
-
Harnessing MUC1 aptamer-targeted nanoparticles for precision medicine in breast cancer.Int J Pharm. 2025 Jun 10;678:125721. doi: 10.1016/j.ijpharm.2025.125721. Epub 2025 May 11. Int J Pharm. 2025. PMID: 40360093 Review.
Cited by
-
Microwave-Assisted Synthesis and Characterization of Poly(L-lysine)-Based Polymer/Carbon Quantum Dot Nanomaterials for Biomedical Purposes.Materials (Basel). 2019 Nov 21;12(23):3825. doi: 10.3390/ma12233825. Materials (Basel). 2019. PMID: 31766363 Free PMC article.
-
Role of diabetes-related inflammation in pancreatic cancer evaluated by aptamer-based detection of circulating tumor cells in a streptozotocin-induced Panc02-transplanted murine model.Ann Hepatobiliary Pancreat Surg. 2025 Aug 31;29(3):343-352. doi: 10.14701/ahbps.25-120. Epub 2025 Aug 5. Ann Hepatobiliary Pancreat Surg. 2025. PMID: 40759530 Free PMC article.
-
Clinical applications of circulating tumor cells in metastasis and therapy.J Hematol Oncol. 2025 Aug 22;18(1):80. doi: 10.1186/s13045-025-01733-y. J Hematol Oncol. 2025. PMID: 40846962 Free PMC article. Review.
-
Targeted drug delivery using an aptamer against shared tumor-specific peptide antigen of MAGE-A3.Cancer Biol Ther. 2021 Jan 2;22(1):12-18. doi: 10.1080/15384047.2020.1833156. Epub 2020 Nov 29. Cancer Biol Ther. 2021. PMID: 33249980 Free PMC article.
-
Aptamer-based diagnostic and therapeutic approaches in animals: Current potential and challenges.Saudi J Biol Sci. 2021 Sep;28(9):5081-5093. doi: 10.1016/j.sjbs.2021.05.031. Epub 2021 May 20. Saudi J Biol Sci. 2021. PMID: 34466086 Free PMC article. Review.
References
-
- Thege F.L., Lannin T.B., Saha T.N., Tsai S., Kochman M.L., Hollingsworth M.A., Rhim A.D., Kirby B.J. Microfluidic immunocapture of circulating pancreatic cells using parallel EpCAM and MUC1 capture: Characterization, optimization and downstream analysis. Lab Chip. 2014;21:1775–1784. doi: 10.1039/C4LC00041B. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

