1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing
- PMID: 27886092
- PMCID: PMC6273535
- DOI: 10.3390/molecules21111600
1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing
Abstract
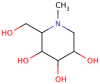
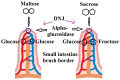
1-Deoxynojirimycin (DNJ, C₆H13NO₄, 163.17 g/mol), an alkaloid azasugar or iminosugar, is a biologically active natural compound that exists in mulberry leaves and Commelina communis (dayflower) as well as from several bacterial strains such as Bacillus and Streptomyces species. Deoxynojirimycin possesses antihyperglycemic, anti-obesity, and antiviral features. Therefore, the aim of this detailed review article is to summarize the existing knowledge on occurrence, extraction, purification, determination, chemistry, and bioactivities of DNJ, so that researchers may use it to explore future perspectives of research on DNJ. Moreover, possible molecular targets of DNJ will also be investigated using suitable in silico approach.
Keywords: Bacillus; anti-obesity; antihyperglycemic; antiviral; fermentation; iminosugar; molecular targets; mulberry; silkworms.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Pearson M.S.M., Mathe-Allainmat M., Fargeas V., Lebreton J. Recent Advances in the Total Synthesis of Piperidine Azasugars. Annalen Der Chemie Und Pharmacie. 2005;36:2159–2191.
-
- Zechel D.L., Withers S.G. Glycosidase mechanisms: Anatomy of a finely tuned catalyst. Acc. Chem. Res. 2000;33:11–18. - PubMed
-
- Yagi M., Kouno T., Aoyagi Y., Murai H. The structure of moranoline, a piperidine alkaloid from Morus species. J. Agric. Chem. Soc. Jpn. 1976;50:571–572.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

