CXCR4/CXCL12 axis counteracts hematopoietic stem cell exhaustion through selective protection against oxidative stress
- PMID: 27886253
- PMCID: PMC5122894
- DOI: 10.1038/srep37827
CXCR4/CXCL12 axis counteracts hematopoietic stem cell exhaustion through selective protection against oxidative stress
Abstract
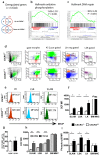
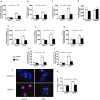
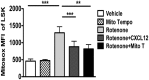
Hematopoietic stem cells (HSCs) undergo self-renewal to maintain hematopoietic homeostasis for lifetime, which is regulated by the bone marrow (BM) microenvironment. The chemokine receptor CXCR4 and its ligand CXCL12 are critical factors supporting quiescence and BM retention of HSCs. Here, we report an unknown function of CXCR4/CXCL12 axis in the protection of HSCs against oxidative stress. Disruption of CXCR4 receptor in mice leads to increased endogenous production of reactive oxygen species (ROS), resulting in p38 MAPK activation, increased DNA double-strand breaks and apoptosis leading to marked reduction in HSC repopulating potential. Increased ROS levels are directly responsible for exhaustion of the HSC pool and are not linked to loss of quiescence of CXCR4-deficient HSCs. Furthermore, we report that CXCL12 has a direct rescue effect on oxidative stress-induced HSC damage at the mitochondrial level. These data highlight the importance of CXCR4/CXCL12 axis in the regulation of lifespan of HSCs by limiting ROS generation and genotoxic stress.
Figures




Similar articles
-
Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells.Nat Med. 2006 Apr;12(4):446-51. doi: 10.1038/nm1388. Epub 2006 Mar 26. Nat Med. 2006. PMID: 16565722
-
Enhanced functional response to CXCL12/SDF-1 through retroviral overexpression of CXCR4 on M07e cells: implications for hematopoietic stem cell transplantation.Stem Cells Dev. 2006 Jun;15(3):325-33. doi: 10.1089/scd.2006.15.325. Stem Cells Dev. 2006. PMID: 16846371
-
CXCR4 is required for the quiescence of primitive hematopoietic cells.J Exp Med. 2008 Apr 14;205(4):777-83. doi: 10.1084/jem.20072513. Epub 2008 Mar 31. J Exp Med. 2008. PMID: 18378795 Free PMC article.
-
Concise Review: CXCR4/CXCL12 Signaling in Immature Hematopoiesis--Lessons From Pharmacological and Genetic Models.Stem Cells. 2015 Aug;33(8):2391-9. doi: 10.1002/stem.2054. Epub 2015 May 25. Stem Cells. 2015. PMID: 25966814 Review.
-
Innate immunity derived factors as external modulators of the CXCL12-CXCR4 axis and their role in stem cell homing and mobilization.Theranostics. 2013;3(1):3-10. doi: 10.7150/thno.4621. Epub 2013 Jan 12. Theranostics. 2013. PMID: 23382780 Free PMC article. Review.
Cited by
-
Supplementation of SDF1 during Pig Oocyte In Vitro Maturation Improves Subsequent Embryo Development.Molecules. 2022 Oct 12;27(20):6830. doi: 10.3390/molecules27206830. Molecules. 2022. PMID: 36296422 Free PMC article.
-
Mesenchymal Stem Cell Secretion of SDF-1α Modulates Endothelial Function in Dilated Cardiomyopathy.Front Physiol. 2019 Sep 24;10:1182. doi: 10.3389/fphys.2019.01182. eCollection 2019. Front Physiol. 2019. PMID: 31616309 Free PMC article.
-
The Triterpenoid Nrf2 Activator, CDDO-Me, Decreases Neutrophil Senescence in a Murine Model of Joint Damage.Int J Mol Sci. 2023 May 15;24(10):8775. doi: 10.3390/ijms24108775. Int J Mol Sci. 2023. PMID: 37240121 Free PMC article.
-
CHIP: a clonal odyssey of the bone marrow niche.J Clin Invest. 2024 Aug 1;134(15):e180068. doi: 10.1172/JCI180068. J Clin Invest. 2024. PMID: 39087468 Free PMC article. Review.
-
Humanized zebrafish enhance human hematopoietic stem cell survival and promote acute myeloid leukemia clonal diversity.Haematologica. 2020 Oct 1;105(10):2391-2399. doi: 10.3324/haematol.2019.223040. Haematologica. 2020. PMID: 33054079 Free PMC article.
References
-
- D’Autréaux B. & Toledano M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 (2007). - PubMed
-
- Yahata T. et al.. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood 118, 2941–50 (2011). - PubMed
-
- Adam-Vizi V. & Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 27, 639–45 (2006). - PubMed
-
- Rigoulet M., Yoboue E. D. & Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxid. Redox Signal. 14, 459–68 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

