First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers
- PMID: 27886407
- PMCID: PMC5324684
- DOI: 10.1002/mds.26878
First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers
Abstract
Background: α-Synuclein is a major component of pathologic inclusions that characterize Parkinson's disease. PRX002 is an antibody that targets α-synuclein, and its murine parent antibody 9E4 has been shown in preclinical studies to reduce α-synuclein pathology and to protect against cognitive and motor deteriorations and progressive neurodegeneration in human α-synuclein transgenic mice.
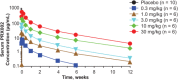
Methods: This first-in-human, randomized, double-blind, placebo-controlled, phase 1 study assessed the impact of PRX002 administered to 40 healthy participants in 5 ascending-dose cohorts (n = 8/cohort) in which participants were randomly assigned to receive a single intravenous infusion of study drug (0.3, 1, 3, 10, or 30 mg/kg; n = 6/cohort) or placebo (n = 2/cohort).
Results: PRX002 demonstrated favorable safety, tolerability, and pharmacokinetic profiles at all doses tested, with no immunogenicity. No serious adverse events, discontinuations as a result of adverse events, or dose-limiting toxicities were reported. Serum PRX002 exposure was dose proportional; the average terminal half-life across all doses was 18.2 days. A significant dose-dependent reduction in free serum α-synuclein (unbound to PRX002) was apparent within 1 hour after PRX002 administration, whereas total α-synuclein (free plus bound) increased dose-dependently, presumably because of the expected change in kinetics following antibody binding.
Conclusions: This study demonstrates that serum α-synuclein can be safely modulated in a dose-dependent manner after single intravenous infusions of an anti-α-synuclein antibody. These findings support continued development of PRX002, including further characterization of its safety, tolerability, pharmacokinetics, and pharmacodynamic effects in the central nervous system in patients with Parkinson's disease. © 2016 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Trial registration: ClinicalTrials.gov NCT02157714.
Keywords: Parkinson's disease; clinical trial; protein aggregation; protein misfolding; synucleinopathy.
© 2016 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Figures
Comment in
-
Targeting α-Synuclein as a therapy for Parkinson's disease: The battle begins.Mov Disord. 2017 Feb;32(2):203-207. doi: 10.1002/mds.26935. Mov Disord. 2017. PMID: 28218461 No abstract available.
References
-
- Stocchi F, Martinez‐Martin P, Reichmann H. Quality of life in Parkinson's disease–patient, clinical and research perspectives. http://www.touchneurology.com/articles/quality-life-parkinson-s-disease-.... Accessed July 5, 2016.
-
- Sobell JM, Foley P, Toth D, et al. Effects of apremilast on pruritus and skin discomfort/pain correlate with improvements in quality of life in patients with moderate to severe plaque psoriasis. Acta Derm Venereol 2016;96:514–520. - PubMed
-
- Nussbaum RL, Ellis CE. Alzheimer's disease and Parkinson's disease. N Engl J Med 2003;348:1356–1364. - PubMed
-
- Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord 2013;28:311–318. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous