Inflammatory Stimuli Increase Progesterone Receptor-A Stability and Transrepressive Activity in Myometrial Cells
- PMID: 27886516
- PMCID: PMC5412979
- DOI: 10.1210/en.2016-1537
Inflammatory Stimuli Increase Progesterone Receptor-A Stability and Transrepressive Activity in Myometrial Cells
Abstract
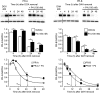
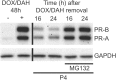
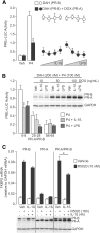
The steroid hormone progesterone acting via the nuclear progesterone receptor (PR) isoforms, progesterone receptor A (PR-A) and progesterone receptor B (PR-B), is essential for the maintenance of uterine quiescence during pregnancy. Inhibition of PR signaling augments uterine contractility and induces labor. Human parturition is thought to be triggered by modulation of PR signaling in myometrial cells to induce a functional progesterone withdrawal. One mechanism for functional progesterone withdrawal is increased abundance of PR-A, which decreases progesterone responsiveness by inhibiting the transcriptional activity of PR-B. Human parturition also involves tissue-level inflammation within the myometrium. This study examined the control of PR-A abundance and transrepressive activity in myometrial cells and the role of the inflammatory stimuli in the form of interleukin-1β (IL-1β) and lipopolysaccharide (LPS) in these processes. We found that abundance of PR-A was markedly increased by progesterone and by exposure to IL-1β and LPS via posttranslational mechanisms involving increased PR-A protein stability. In contrast, progesterone decreased abundance of PR-B by increasing its rate of degradation. Together, progesterone and proinflammatory stimuli induced a PR-A-dominant state in myometrial cells similar to that observed in term laboring myometrium. IL-1β and LPS also increased the capacity for PR-A to inhibit the transcriptional activity of PR-B. Taken together, our data suggest that proinflammatory stimuli increase the steady-state levels of PR-A and its transrepressive activity in myometrial cells and support the hypothesis that tissue-level inflammation triggers parturition by inducing PR-A-mediated functional progesterone withdrawal.
Copyright © 2017 by the Endocrine Society.
Figures




Similar articles
-
Control of Progesterone Receptor-A Transrepressive Activity in Myometrial Cells: Implications for the Control of Human Parturition.Reprod Sci. 2018 Feb;25(2):214-221. doi: 10.1177/1933719117716775. Epub 2017 Jul 3. Reprod Sci. 2018. PMID: 28671036 Free PMC article.
-
Human Parturition Involves Phosphorylation of Progesterone Receptor-A at Serine-345 in Myometrial Cells.Endocrinology. 2016 Nov;157(11):4434-4445. doi: 10.1210/en.2016-1654. Epub 2016 Sep 21. Endocrinology. 2016. PMID: 27653036 Free PMC article.
-
Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A.J Clin Endocrinol Metab. 2007 May;92(5):1927-33. doi: 10.1210/jc.2007-0077. Epub 2007 Mar 6. J Clin Endocrinol Metab. 2007. PMID: 17341556
-
Myometrial progesterone responsiveness and the control of human parturition.J Soc Gynecol Investig. 2004 May;11(4):193-202. doi: 10.1016/j.jsgi.2003.12.004. J Soc Gynecol Investig. 2004. PMID: 15120691 Review.
-
The role of progesterone receptor isoforms in the myometrium.J Steroid Biochem Mol Biol. 2022 Nov;224:106160. doi: 10.1016/j.jsbmb.2022.106160. Epub 2022 Aug 3. J Steroid Biochem Mol Biol. 2022. PMID: 35931328 Free PMC article. Review.
Cited by
-
Regulatory mechanisms of HMGB1 and its receptors in polycystic ovary syndrome-driven gravid uterine inflammation.FEBS J. 2023 Apr;290(7):1874-1906. doi: 10.1111/febs.16678. Epub 2022 Dec 8. FEBS J. 2023. PMID: 36380688 Free PMC article.
-
Progesterone receptor isoform B regulates the Oxtr-Plcl2-Trpc3 pathway to suppress uterine contractility.Proc Natl Acad Sci U S A. 2021 Mar 16;118(11):e2011643118. doi: 10.1073/pnas.2011643118. Proc Natl Acad Sci U S A. 2021. PMID: 33707208 Free PMC article.
-
Transcriptome meta-analysis reveals differences of immune profile between eutopic endometrium from stage I-II and III-IV endometriosis independently of hormonal milieu.Sci Rep. 2020 Jan 15;10(1):313. doi: 10.1038/s41598-019-57207-y. Sci Rep. 2020. PMID: 31941945 Free PMC article.
-
Enhanced drug delivery to the reproductive tract using nanomedicine reveals therapeutic options for prevention of preterm birth.Sci Transl Med. 2021 Jan 13;13(576):eabc6245. doi: 10.1126/scitranslmed.abc6245. Sci Transl Med. 2021. PMID: 33441428 Free PMC article.
-
Multiomic immune clockworks of pregnancy.Semin Immunopathol. 2020 Aug;42(4):397-412. doi: 10.1007/s00281-019-00772-1. Epub 2020 Feb 4. Semin Immunopathol. 2020. PMID: 32020337 Free PMC article. Review.
References
-
- Liggins GC. Endocrinology of parturition. In: Novy MJ, Resko JA, eds. Fetal Endocrinology. New York, NY: Academic Press, Inc.; 1981:211–237.
-
- Young IR, Renfree MB, Mesiano S, Shaw G, Jenkin G, Smith R. The comparative physiology of parturition in mammals: Hormones and parturition in mammals. In: Norris D, Lopez K, eds. Hormones and Reproduction in Vertebrates, volume 5 London: Academic Press; 2010:95–116.
-
- Avrech OM, Golan A, Weinraub Z, Bukovsky I, Caspi E. Mifepristone (RU486) alone or in combination with a prostaglandin analogue for termination of early pregnancy: a review. Fertil Steril. 1991;56(3):385–393. - PubMed
-
- Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112(8):1095–1100. - PubMed
-
- Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod. 2001;7(9):875–879. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

