Hyaluronan-Derived Swelling of Solid Tumors, the Contribution of Collagen and Cancer Cells, and Implications for Cancer Therapy
- PMID: 27886639
- PMCID: PMC5122704
- DOI: 10.1016/j.neo.2016.10.001
Hyaluronan-Derived Swelling of Solid Tumors, the Contribution of Collagen and Cancer Cells, and Implications for Cancer Therapy
Abstract
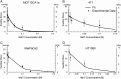
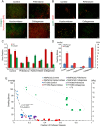
Despite the important role that mechanical forces play in tumor growth and therapy, the contribution of swelling to tumor mechanopathology remains unexplored. Tumors rich in hyaluronan exhibit a highly negative fixed charge density. Repulsive forces among these negative charges as well as swelling of cancer cells due to regulation of intracellular tonicity can cause tumor swelling and development of stress that might compress blood vessels, compromising tumor perfusion and drug delivery. Here, we designed an experimental strategy, using four orthotopic tumor models, to measure swelling stress and related swelling to extracellular matrix components, hyaluronan and collagen, as well as to tumor perfusion. Subsequently, interventions were performed to measure tumor swelling using matrix-modifying enzymes (hyaluronidase and collagenase) and by repurposing pirfenidone, an approved antifibrotic drug. Finally, in vitro experiments on cancer cell spheroids were performed to identify their contribution to tissue swelling. Swelling stress was measured in the range of 16 to 75 mm Hg, high enough to cause vessel collapse. Interestingly, while depletion of hyaluronan decreased swelling, collagen depletion had the opposite effect, whereas the contribution of cancer cells was negligible. Furthermore, histological analysis revealed the same linear correlation between tumor swelling and the ratio of hyaluronan to collagen content when data from all tumor models were combined. Our data further revealed an inverse relation between tumor perfusion and swelling, suggesting that reduction of swelling decompresses tumor vessels. These results provide guidelines for emerging therapeutic strategies that target the tumor microenvironment to alleviate intratumoral stresses and improve vessel functionality and drug delivery.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005–1060. - PubMed
-
- Alexandrakis G, Brown EB, Tong RT, McKee TD, Campbell RB, Boucher Y, Jain RK. Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors. Nat Med. 2004;10:203–207. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

