Integral strategy to supportive care in breast cancer survivors through occupational therapy and a m-health system: design of a randomized clinical trial
- PMID: 27887610
- PMCID: PMC5124301
- DOI: 10.1186/s12911-016-0394-0
Integral strategy to supportive care in breast cancer survivors through occupational therapy and a m-health system: design of a randomized clinical trial
Abstract
Background: Technological support using e-health mobile applications (m-health) is a promising strategy to improve the adherence to healthy lifestyles in breast cancer survivors (excess in energy intake or low physical activity are determinants of the risk of recurrence, second cancers and cancer mortality). Moreover, cancer rehabilitation programs supervised by health professionals are needed due to the inherent characteristics of these breast cancer patients. Our main objective is to compare the clinical efficacy of a m-health lifestyle intervention system alone versus an integral strategy to improve Quality of Life in breast cancer survivors.
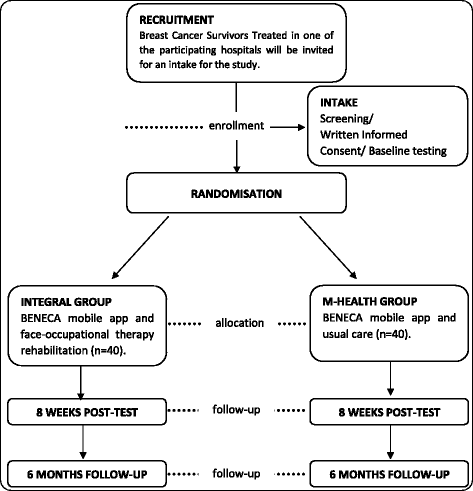
Methods: This therapeutic superiority study will use a two-arm, assessor blinded parallel RCT design. Women will be eligible if: they are diagnosed of stage I, II or III-A breast cancer; are between 25 and 75 years old; have a Body Mass Index > 25 kg/m2; they have basic ability to use mobile apps; they had completed adjuvant therapy except for hormone therapy; and they have some functional shoulder limitations. Participants will be randomized to one of the following groups: integral group will use a mobile application (BENECA APP) and will receive a face-to-face rehabilitation (8-weeks); m-health group will use the BENECA app for 2-months and will received usual care information. Study endpoints will be assessed after 8 weeks and 6 months. The primary outcome will be Quality of Life measured by The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core and breast module. The secondary outcomes: body composition; upper-body functionality (handgrip, Disability of the Arm, Shoulder and Hand questionnaire, goniometry); cognitive function (Wechsler Adult Intelligence Scale, Trail Making Test); anxiety and depression (Hospital Anxiety and Depression Scale); physical fitness (Short version of the Minnesota Leisure Time Physical Activity Questionnaire, Self-Efficacy Scale for Physical Activity); accelerometry and lymphedema.
Discussion: This study has been designed to seek to address the new needs for support and treatment of breast cancer survivors, reflecting the emerging need to merge new low cost treatment options with much-needed involvement of health professionals in this type of patients.
Trial registration: ClinicalTrials.gov Identifier: NCT02817724 (date of registration: 22/06/2016).
Keywords: Breast; Mobile applications; Neoplasms; Occupational therapy; Quality of life.
Figures
Similar articles
-
Telehealth system (e-CUIDATE) to improve quality of life in breast cancer survivors: rationale and study protocol for a randomized clinical trial.Trials. 2013 Jun 22;14:187. doi: 10.1186/1745-6215-14-187. Trials. 2013. PMID: 23799886 Free PMC article. Clinical Trial.
-
Mobile health and supervised rehabilitation versus mobile health alone in breast cancer survivors: Randomized controlled trial.Ann Phys Rehabil Med. 2020 Jul;63(4):316-324. doi: 10.1016/j.rehab.2019.07.007. Epub 2019 Aug 24. Ann Phys Rehabil Med. 2020. PMID: 31454561 Clinical Trial.
-
A Mobile System to Improve Quality of Life Via Energy Balance in Breast Cancer Survivors (BENECA mHealth): Prospective Test-Retest Quasiexperimental Feasibility Study.JMIR Mhealth Uhealth. 2019 Jun 25;7(6):e14136. doi: 10.2196/14136. JMIR Mhealth Uhealth. 2019. PMID: 31237570 Free PMC article.
-
The Underutilization of Rehabilitation to Treat Physical Impairments in Breast Cancer Survivors.PM R. 2017 Sep;9(9S2):S317-S323. doi: 10.1016/j.pmrj.2017.05.010. PM R. 2017. PMID: 28942906 Review.
-
Mobile Health Applications, Cancer Survivors, and Lifestyle Modification: An Integrative Review.Comput Inform Nurs. 2021 Jun 2;39(11):755-763. doi: 10.1097/CIN.0000000000000781. Comput Inform Nurs. 2021. PMID: 34074873 Free PMC article. Review.
Cited by
-
Patient Experiences of Rehabilitation and the Potential for an mHealth System with Biofeedback After Breast Cancer Surgery: Qualitative Study.JMIR Mhealth Uhealth. 2020 Jul 29;8(7):e19721. doi: 10.2196/19721. JMIR Mhealth Uhealth. 2020. PMID: 32687476 Free PMC article.
-
Development and preliminary evaluation of a tele-rehabilitation exercise system using computer-generated animation.Fujita Med J. 2022 Nov;8(4):114-120. doi: 10.20407/fmj.2021-020. Epub 2022 Jan 25. Fujita Med J. 2022. PMID: 36415828 Free PMC article.
-
Development of an e-health app to support women prescribed adjuvant endocrine therapy after treatment for breast cancer.Patient Prefer Adherence. 2018 Dec 11;12:2639-2647. doi: 10.2147/PPA.S187692. eCollection 2018. Patient Prefer Adherence. 2018. PMID: 30587936 Free PMC article.
-
Usability of a mobile phone application to enhance activities of daily living in occupational therapy services for breast cancer survivors.Hong Kong J Occup Ther. 2023 Dec;36(2):128-140. doi: 10.1177/15691861231206489. Epub 2023 Oct 27. Hong Kong J Occup Ther. 2023. PMID: 38027046 Free PMC article.
-
Telerehabilitation and Monitoring Physical Activity in Patient with Breast Cancer: Systematic Review.Iran J Nurs Midwifery Res. 2022 Jan 25;27(1):8-17. doi: 10.4103/ijnmr.ijnmr_472_20. eCollection 2022 Jan-Feb. Iran J Nurs Midwifery Res. 2022. PMID: 35280190 Free PMC article. Review.
References
-
- Jemal A, Vineis P, Bray F, et al. The Cancer Atlas. In: American Center Society, editor. 2nd ed. Atlanta: Ahmedin Jemal; 2014.
-
- Ganz PA, Yip CH, Gralow JR, Distelhorst SR, Albain KS, Andersen BL, et al. Supportive care after curative treatment for breast cancer (survivorship care): resource allocations in low- and middle-income countries. A Breast Health Global Initiative 2013 consensus statement. Breast. 2013;22:606–15. doi: 10.1016/j.breast.2013.07.049. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical