A Pilot Study of a Multimodal Treatment Paradigm to Accelerate Drug Evaluations in Early-stage Metastatic Prostate Cancer
- PMID: 27888148
- PMCID: PMC5468169
- DOI: 10.1016/j.urology.2016.10.044
A Pilot Study of a Multimodal Treatment Paradigm to Accelerate Drug Evaluations in Early-stage Metastatic Prostate Cancer
Abstract
Objective: To evaluate a multimodal strategy aimed at treating all sites of disease that provides a rapid readout of success or failure in men presenting with non-castrate metastatic prostate cancers that are incurable with single modality therapy.
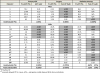
Materials and methods: Twenty selected men with oligometastatic M1a (extrapelvic nodal disease) or M1b (bone disease) at diagnosis were treated using a multimodal approach that included androgen deprivation, radical prostatectomy plus pelvic lymphadenectomy (retroperitoneal lymphadenectomy in the presence of clinically positive retroperitoneal nodes), and stereotactic body radiotherapy to osseous disease or the primary site. Outcomes of each treatment were assessed sequentially. Androgen deprivation was discontinued in responding patients. The primary end point was an undetectable prostate-specific antigen (PSA) after testosterone recovery. The goal was to eliminate all detectable disease.
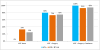
Results: Each treatment modality contributed to the outcome: 95% of the cohort achieved an undetectable PSA with multimodal treatment, including 25% of patients after androgen deprivation alone and an additional 50% and 20% after surgery and radiotherapy, respectively. Overall, 20% of patients (95% confidence interval: 3%-38%) achieved the primary end point, which persisted for 5, 6, 27+ , and 46+ months. All patients meeting the primary end point had been classified with M1b disease at presentation.
Conclusion: A sequentially applied multimodal treatment strategy can eliminate detectable disease in selected patients with metastatic spread at diagnosis. The end point of undetectable PSA after testosterone recovery should be considered when evaluating new approaches to rapidly set priorities for large-scale testing in early metastatic disease states and to shift the paradigm from palliation to cure.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Editorial Comment.Urology. 2017 Apr;102:171-172. doi: 10.1016/j.urology.2016.10.045. Epub 2017 Mar 6. Urology. 2017. PMID: 28279478 No abstract available.
References
-
- Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073. - PubMed
-
- Roach M, 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. - PubMed
-
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–308. - PubMed
-
- Klotz LH, Goldenberg SL, Jewett MA, et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170:791–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

