Reversed-phase separation methods for glycan analysis
- PMID: 27888305
- PMCID: PMC5203856
- DOI: 10.1007/s00216-016-0073-0
Reversed-phase separation methods for glycan analysis
Abstract
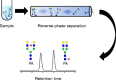
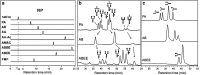
Reversed-phase chromatography is a method that is often used for glycan separation. For this, glycans are often derivatized with a hydrophobic tag to achieve retention on hydrophobic stationary phases. The separation and elution order of glycans in reversed-phase chromatography is highly dependent on the hydrophobicity of the tag and the contribution of the glycan itself to the retention. The contribution of the different monosaccharides to the retention strongly depends on the position and linkage, and isomer separation may be achieved. The influence of sialic acids and fucoses on the retention of glycans is still incompletely understood and deserves further study. Analysis of complex samples may come with incomplete separation of glycan species, thereby complicating reversed-phase chromatography with fluorescence or UV detection, whereas coupling with mass spectrometry detection allows the resolution of complex mixtures. Depending on the column properties, eluents, and run time, separation of isomeric and isobaric structures can be accomplished with reversed-phase chromatography. Alternatively, porous graphitized carbon chromatography and hydrophilic interaction liquid chromatography are also able to separate isomeric and isobaric structures, generally without the necessity of glycan labeling. Hydrophilic interaction liquid chromatography, porous graphitized carbon chromatography, and reversed-phase chromatography all serve different research purposes and thus can be used for different research questions. A great advantage of reversed-phase chromatography is its broad distribution as it is used in virtually every bioanalytical research laboratory, making it an attracting platform for glycan analysis. Graphical Abstract Glycan isomer separation by reversed phase liquid chromatography.
Keywords: Glycan; Liquid chromatography; Reversed phase; Separation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Varki A, Esko JD, Colley KJ, et al. Cellular organization of glycosylation. In: Varki A, Cummings R, Esko J, Freeze HH, Goldsmith HW, Bertozzi CR, et al., editors. Essentials of glycobiology. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2009.
-
- Bertozzi C, Rabuka D, et al. Structural basis of glycan diversity. In: Varki A, Cummings R, Esko J, Freeze HH, Goldsmith HW, Bertozzi CR, et al., editors. Essentials of glycobiology. 2. New York: Cold Spring Harbor Laboratory Press; 2009. - PubMed
-
- Geyer H, Geyer R. Strategies for analysis of glycoprotein glycosylation. Biochim Biophys Acta. 1764;2006:1853–1869. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

