Chitosan Nanolayered Cisplatin-Loaded Lipid Nanoparticles for Enhanced Anticancer Efficacy in Cervical Cancer
- PMID: 27888498
- PMCID: PMC5124019
- DOI: 10.1186/s11671-016-1698-9
Chitosan Nanolayered Cisplatin-Loaded Lipid Nanoparticles for Enhanced Anticancer Efficacy in Cervical Cancer
Abstract
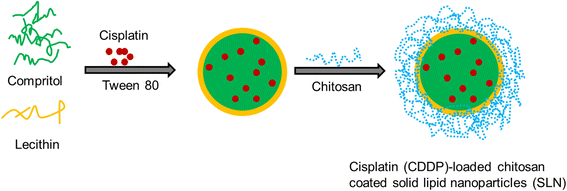
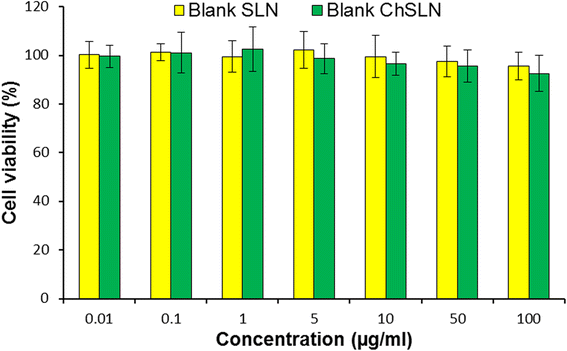
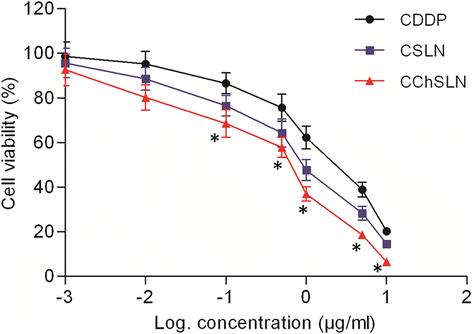
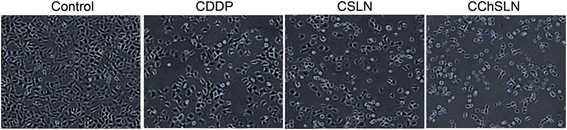
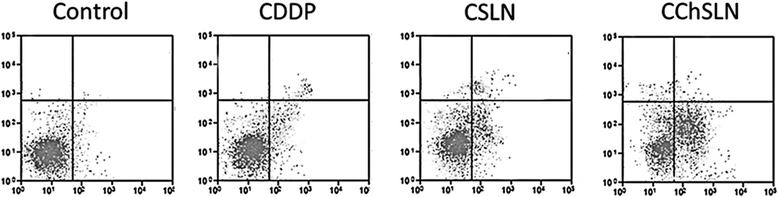
In this study, cisplatin (CDDP)-loaded chitosan-coated solid lipid nanoparticles (SLN) was successfully formulated to treat HeLa cervical carcinoma. The formulation nanoparticles were nanosized and exhibited a controlled release of drug in physiological conditions. The blank nanoparticles exhibited an excellent biocompatibility profile indicating its suitability for cancer targeting. The incorporation of CDDP in SLN remarkably increased the cancer cell death as evident from the MTT assay. Importantly, CDDP-loaded chitosan-coated SLN (CChSLN) significantly (P < 0.05) decreased the viability of cancer cells even at low concentration. The higher cytotoxicity potential of CChSLN was attributed to the higher cellular uptake as well as the sustained drug release manner in comparison with CSLN. Consistent with the cytotoxicity assay, CChSLN showed the lowest IC50 value of 0.6125 μg/ml while CSLN presented 1.156 μg/ml. CChSLN showed a significantly higher apoptosis in cancer cells compared to that of CSLN and CDDP, which is attributed to the better internalization of nanocarriers and controlled release of anticancer drugs in the intracellular environment. Our findings suggest that this new formulation could be a promising alternative for the treatment of cervical cancers. These findings are encouraging us to continue our research, with a more extended investigation of cellular response in real time and in animal models.
Keywords: Antitumor efficacy; Cervical cancers; Chitosan; Cisplatin; Solid lipid nanoparticles.
Figures




References
-
- IARC. Monographs on the evaluation of carcinogenic risks to humans: human papillomaviruses, vol. 90. http://monographs.iarc.fr/. Accessed 10 May 2016.
-
- WHO (World Health Organization). Human papillomaviruses. http://www.w0ho.int/vaccine_research/diseases/viral_cancers/en/index3.html. Accessed 10 May 2016.
-
- Ma Y, Huang L, Song C, Zeng X, Liu G, Mei L. Nanoparticleformulation of poly(ε-caprolactone-co-lactide)-d-α-tocopheryl polyethyleneglycol 1000 succinate random copolymer for cervical cancer treatment. Polymer. 2010;51:5952–5959. doi: 10.1016/j.polymer.2010.10.029. - DOI
-
- Kjaer SK, van den Brule AJ, Paull G, Svare EI, Sherman ME, Thomsen BL, Suntum M, Bock JE, Poll PA, Meijer CJ. Type specific persistence ofhigh risk human papillomavirus (HPV) as indicator of high grade cervicalsquamous intraepithelial lesions in young women: population basedprospective follow up study. BMJ. 2002;325:572. doi: 10.1136/bmj.325.7364.572. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

