Structural and electronic properties of two-dimensional stanene and graphene heterostructure
- PMID: 27888499
- PMCID: PMC5124025
- DOI: 10.1186/s11671-016-1731-z
Structural and electronic properties of two-dimensional stanene and graphene heterostructure
Abstract
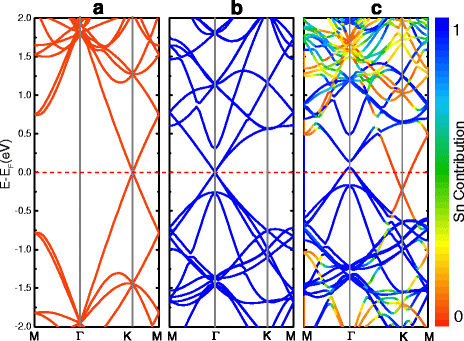
Structural and electronic properties of two-dimensional stanene and graphene heterostructure (Sn/G) are studied by using first-principles calculations. Various supercell models are constructed in order to reduce the strain induced by the lattice mismatch. The results show that stanene interacts overall weakly with graphene via van der Waals (vdW) interactions. Multiple phases of different crystalline orientation of stanene and graphene could coexist at room temperature. Moreover, interlayer interactions in stanene and graphene heterostructure can induce tunable band gaps at stanene's Dirac point, and weak p-type and n-type doping of stanene and graphene, respectively, generating a small amount of electron transfer from stanene to graphene. Interestingly, for model [Formula: see text] , there emerges a band gap about 34 meV overall the band structure, indicating it shows semiconductor feature.
Keywords: First-principles; Graphene; Heterostructure; Stanene; Structural properties.
Figures







References
-
- Wang FQ, Yu J, Wang Q, Kawazoe Y, Jena P. Lattice thermal conductivity of penta-graphene. Carbon. 2016;105:424–429. doi: 10.1016/j.carbon.2016.04.054. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

