cAMP-independent signal pathways stimulate hyphal morphogenesis in Candida albicans
- PMID: 27888610
- PMCID: PMC5323341
- DOI: 10.1111/mmi.13588
cAMP-independent signal pathways stimulate hyphal morphogenesis in Candida albicans
Abstract
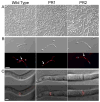
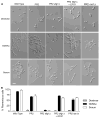
The fungal pathogen Candida albicans can transition from budding to hyphal growth, which promotes biofilm formation and invasive growth into tissues. Stimulation of adenylyl cyclase to form cAMP induces hyphal morphogenesis. The failure of cells lacking adenylyl cyclase (cyr1Δ) to form hyphae has suggested that cAMP signaling is essential for hyphal growth. However, cyr1Δ mutants also grow slowly and have defects in morphogenesis, making it unclear whether hyphal inducers must stimulate cAMP, or if normal basal levels of cAMP are required to maintain cellular health needed for hyphal growth. Interestingly, supplementation of cyr1Δ cells with low levels of cAMP enabled them to form hyphae in response to the inducer N-acetylglucosamine (GlcNAc), suggesting that a basal level of cAMP is sufficient for stimulation. Furthermore, we isolated faster-growing cyr1Δ pseudorevertant strains that can be induced to form hyphae even though they lack cAMP. The pseudorevertant strains were not induced by CO2 , consistent with reports that CO2 directly stimulates adenylyl cyclase. Mutational analysis showed that induction of hyphae in a pseudorevertant strain was independent of RAS1, but was dependent on the EFG1 transcription factor that acts downstream of protein kinase A. Thus, cAMP-independent signals contribute to the induction of hyphal responses.
© 2016 John Wiley & Sons Ltd.
Figures





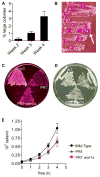
Similar articles
-
Regulation of Hyphal Growth and N-Acetylglucosamine Catabolism by Two Transcription Factors in Candida albicans.Genetics. 2017 May;206(1):299-314. doi: 10.1534/genetics.117.201491. Epub 2017 Mar 27. Genetics. 2017. PMID: 28348062 Free PMC article.
-
Integrative multi-omics profiling reveals cAMP-independent mechanisms regulating hyphal morphogenesis in Candida albicans.PLoS Pathog. 2021 Aug 16;17(8):e1009861. doi: 10.1371/journal.ppat.1009861. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34398936 Free PMC article.
-
Integration of the tricarboxylic acid (TCA) cycle with cAMP signaling and Sfl2 pathways in the regulation of CO2 sensing and hyphal development in Candida albicans.PLoS Genet. 2017 Aug 7;13(8):e1006949. doi: 10.1371/journal.pgen.1006949. eCollection 2017 Aug. PLoS Genet. 2017. PMID: 28787458 Free PMC article.
-
N-acetylglucosamine-mediated morphological transition in Candida albicans and Candida tropicalis.Curr Genet. 2021 Apr;67(2):249-254. doi: 10.1007/s00294-020-01138-z. Epub 2021 Jan 2. Curr Genet. 2021. PMID: 33388851 Review.
-
Signaling through protein kinases and transcriptional regulators in Candida albicans.Crit Rev Microbiol. 2003;29(3):259-75. doi: 10.1080/713610451. Crit Rev Microbiol. 2003. PMID: 14582620 Review.
Cited by
-
Regulation of Hyphal Growth and N-Acetylglucosamine Catabolism by Two Transcription Factors in Candida albicans.Genetics. 2017 May;206(1):299-314. doi: 10.1534/genetics.117.201491. Epub 2017 Mar 27. Genetics. 2017. PMID: 28348062 Free PMC article.
-
Metabolic and Phenotypic Changes Induced during N-Acetylglucosamine Signalling in the Fungal Pathogen Candida albicans.Biomedicines. 2023 Jul 14;11(7):1997. doi: 10.3390/biomedicines11071997. Biomedicines. 2023. PMID: 37509635 Free PMC article.
-
N-Acetylglucosamine (GlcNAc) Sensing, Utilization, and Functions in Candida albicans.J Fungi (Basel). 2020 Aug 7;6(3):129. doi: 10.3390/jof6030129. J Fungi (Basel). 2020. PMID: 32784532 Free PMC article. Review.
-
From Jekyll to Hyde: The Yeast-Hyphal Transition of Candida albicans.Pathogens. 2021 Jul 7;10(7):859. doi: 10.3390/pathogens10070859. Pathogens. 2021. PMID: 34358008 Free PMC article. Review.
-
Molecular Determinants Involved in Candida albicans Biofilm Formation and Regulation.Mol Biotechnol. 2024 Jul;66(7):1640-1659. doi: 10.1007/s12033-023-00796-x. Epub 2023 Jul 6. Mol Biotechnol. 2024. PMID: 37410258 Review.
References
-
- Bahn YS, Staab J, Sundstrom P. Increased high-affinity phosphodiesterase PDE2 gene expression in germ tubes counteracts CAP1-dependent synthesis of cyclic AMP, limits hypha production and promotes virulence of Candida albicans. Mol Microbiol. 2003;50:391–409. - PubMed
-
- Bai C, Xu XL, Wang HS, Wang YM, Chan FY, Wang Y. Characterization of a hyperactive Cyr1 mutant reveals new regulatory mechanisms for cellular cAMP levels in Candida albicans. Mol Microbiol. 2011;82:879–893. - PubMed
-
- Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol 2006 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

