Suppression of osteopontin inhibits chemically induced hepatic carcinogenesis by induction of apoptosis in mice
- PMID: 27888617
- PMCID: PMC5349983
- DOI: 10.18632/oncotarget.13529
Suppression of osteopontin inhibits chemically induced hepatic carcinogenesis by induction of apoptosis in mice
Abstract
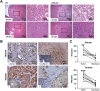
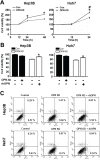
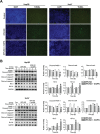
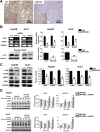
Previous clinical reports have found elevated osteopontin (OPN) levels in tumor tissues to be indicative of greater malignancy in human hepatocellular carcinoma (HCC). However, the role of OPN on carcinogenesis and its underlying mechanism remain unclear. In the present study, we investigated the oncogenic role of OPN in diethylnitrosamine (DEN)-induced hepatic carcinogenesis in mice. The overall incidence of hepatic tumors at 36 weeks was significantly lower in OPN knockout (KO) mice than in wild-type (WT) mice. Apoptosis was significantly enhanced in OPN KO mice, and was accompanied by the downregulation of epidermal growth factor receptor (EGFR). In the in vitro study, OPN suppression also led to lower mRNA and protein levels of EGFR associated with the downregulation of c-Jun in Hep3B and Huh7 human HCC cells lines, which resulted in increased apoptotic cell death in both cell lines. Moreover, a positive correlation was clearly identified between the expression of OPN and EGFR in human HCC tissues. These data demonstrate that the OPN deficiency reduced the incidence of chemically induced HCC by suppressing EGFR-mediated anti-apoptotic signaling. An important implication of our findings is that OPN positively contributes to hepatic carcinogenesis.
Keywords: EGFR; apoptosis; c-Jun; hepatocellular carcinoma; osteopontin.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 2013;11
-
- Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Seraq HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–1380. - PubMed
-
- Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, Orsini L, Kim WR, Roberts LR. Factor that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011;9:617–623. - PubMed
-
- Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: A emerging menace. J Hepatol. 2012;56:1384–1391. - PubMed
-
- Ringelhan M, Protzer U. Oncogenic potential of hepatitis B virus encoded proteins. Curr Opin Virol. 2015;14:109–115. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

