Targeting protein kinase CK2 suppresses bladder cancer cell survival via the glucose metabolic pathway
- PMID: 27888634
- PMCID: PMC5349994
- DOI: 10.18632/oncotarget.13571
Targeting protein kinase CK2 suppresses bladder cancer cell survival via the glucose metabolic pathway
Abstract
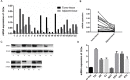
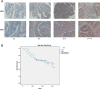
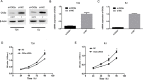
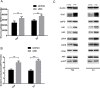
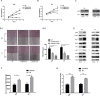
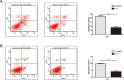
Casein kinase 2 (CK2) is a constitutively active serine/threonine kinase that promotes cell proliferation and resists apoptosis. Elevated CK2 expression has been demonstrated in several solid tumors. The expression of CK2α in bladder cancer was elevated in tumor tissues compared with that in adjacent normal tissues. Amplified expression of CK2α was highly correlated with histological grade in bladder cancer(P = 0.024). Knockdown of CK2α in bladder cancer cell lines resulted in a reduction in tumor aerobic glycolysis, accompanied with lower phosphorylated AKT. Moreover, low CK2α levels suppressed cell growth, and similar results could be reproduced after treatment with CX-4945 with a dose-dependent response. CX-4945 inhibited migration and induced apoptosis. Furthermore, knockdown of CK2α decreased the tumorigenicity of bladder cancer cells in vivo. This study is the first to report that CK2 increases glucose metabolism in human bladder cancer. Blocking CK2 function may provide novel diagnostic and potential therapeutic.
Keywords: CK2α; bladder cancer; glycolysis; oncogene; prognosis.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

Similar articles
-
Systemic administration of antisense oligonucleotides simultaneously targeting CK2α and α' subunits reduces orthotopic xenograft prostate tumors in mice.Mol Cell Biochem. 2011 Oct;356(1-2):21-35. doi: 10.1007/s11010-011-0943-x. Epub 2011 Jul 15. Mol Cell Biochem. 2011. PMID: 21761204 Free PMC article.
-
Induction of apoptosis by antisense CK2 in human prostate cancer xenograft model.Mol Cancer Res. 2004 Dec;2(12):712-21. Mol Cancer Res. 2004. PMID: 15634760
-
Targeting protein kinase CK2 suppresses prosurvival signaling pathways and growth of glioblastoma.Clin Cancer Res. 2013 Dec 1;19(23):6484-94. doi: 10.1158/1078-0432.CCR-13-0265. Epub 2013 Sep 13. Clin Cancer Res. 2013. PMID: 24036851 Free PMC article.
-
Targeting CK2 for cancer therapy.Anticancer Drugs. 2005 Nov;16(10):1037-43. doi: 10.1097/00001813-200511000-00001. Anticancer Drugs. 2005. PMID: 16222144 Review.
-
Metabolic phenotype of bladder cancer.Cancer Treat Rev. 2016 Apr;45:46-57. doi: 10.1016/j.ctrv.2016.03.005. Epub 2016 Mar 8. Cancer Treat Rev. 2016. PMID: 26975021 Review.
Cited by
-
Validation of an LC-MS/MS Method for the Quantification of the CK2 Inhibitor Silmitasertib (CX-4945) in Human Plasma.Molecules. 2022 Apr 7;27(8):2394. doi: 10.3390/molecules27082394. Molecules. 2022. PMID: 35458589 Free PMC article.
-
The role of m6A methylation in therapy resistance in cancer.Mol Cancer. 2023 Jun 1;22(1):91. doi: 10.1186/s12943-023-01782-2. Mol Cancer. 2023. PMID: 37264402 Free PMC article. Review.
-
Sphingosine kinase 1 mediates diabetic renal fibrosis via NF-κB signaling pathway: involvement of CK2α.Oncotarget. 2017 Oct 4;8(51):88988-89004. doi: 10.18632/oncotarget.21640. eCollection 2017 Oct 24. Oncotarget. 2017. PMID: 29179493 Free PMC article.
-
Cancer-type dependent expression of CK2 transcripts.PLoS One. 2017 Dec 4;12(12):e0188854. doi: 10.1371/journal.pone.0188854. eCollection 2017. PLoS One. 2017. PMID: 29206231 Free PMC article.
-
CX-4945 Induces Methuosis in Cholangiocarcinoma Cell Lines by a CK2-Independent Mechanism.Cancers (Basel). 2018 Aug 23;10(9):283. doi: 10.3390/cancers10090283. Cancers (Basel). 2018. PMID: 30142881 Free PMC article.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87–108. - PubMed
-
- Burnett G, Kennedy EP. The enzymatic phosphorylation of proteins. The Journal of biological chemistry. 1954;211:969–980. - PubMed
-
- Duncan JS, Litchfield DW. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim Biophys Acta. 2008;1784:33–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

